
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Aluminum in the Al3 + form can be precipitated as Al (OH) 3, adding ammonia to an aluminum solution, but the precipitate is usually gelatinous and difficult to filter. Precipitation from a homogeneous solution provides a more filterable precipitate. The reaction used in this process is the hydrolysis of urea: (image attached)
Suppose this reaction occurs in a 100.00 mL solution with an initial Al3 + concentration of 1.50x10-3 mol / L. How many moles of urea must be hydrolyzed to supply enough hydroxide to stoichiometrically combine Al3 + and form the precipitate Al (OH) 3?
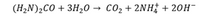
Transcribed Image Text:(H2N)2CO + 3H20 → cO2 + 2NH† + 20H-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(II) carbonate, in concentrated sulfuric acid. The sulfuric acid reacts with the copper(II) carbonate to produce a blue solution of copper(II) sulfate. Scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: Fe(s) + CuSO4(aq) → Cu(s) + FeSO4(aq) Suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. He adds powdered iron to a 200. mL copper(II) sulfate sample from the plant until no more copper will precipitate. He then washes, dries, and weighs the precipitate, and finds that it has a mass of 107. mg. Calculate the original concentration of copper(II) sulfate in the sample. Be sure your answer has the correct number of significant digits. 0 x10 х §arrow_forwardThe concentration of SO42– ions in a 45.0 mL sample of seawater is determined by adding a solution of BaCl2 and precipitating the SO42– as BaSO4. After the precipitate is filtered from the solution, it is dried and weighed. If the mass of BaSO4 recovered is 0.315 g, what is the sulfate concentration of the seawater sample? Express your answer in mmol/L.arrow_forwardA chemistry student needs to standardize a fresh solution of sodium hydroxide. He carefully weighs out 214.mg of oxalic acid H2C2O4 , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in 250.mL of distilled water. The student then titrates the oxalic acid solution with his sodium hydroxide solution. When the titration reaches the equivalence point, the student finds he has used 91.8mL of sodium hydroxide solution. Calculate the molarity of the student's sodium hydroxide solution. Be sure your answer has the correct number of significant digits.arrow_forward
- A) A student completely dissolves 5.16 mol of Ca(OH)2 in water. i) Which equation is the dissociation equation for Ca(OH), in water? (Give the letter choice)| A. Ca(OH) 2 (s) + H 20 ---> CaO(aq) + O 2 (g) + 2 H 2 (g) С. Са(ОН) 2 (s) --> Са 2+ (aq) + 20 2- (aq) + 2H * (aq) В. Cа(ОН) 2 (s) ---> Са 2" (аq) + 2 ОН (аq) D. Ca(ОН) 2 (s) --> CаН 2 (aq) + O 2 (g) ii) How many total moles of ions are released from the 5.16 mol of Ca(OH)2 ? mol of ions B) A sample of FeCl3 is completely dissolved in water. i) Which is the dissociation equation for FeCl3 in water? (Give the letter choice) A. FeCl 3 (s) + H 20 ---> FeCl 2 (aq) + HCI(aq) + OH (aq) C. FeCl (s) ---> Fe 3+ (aд) + Cl 2 (aq) + Cl (aq) 3 В. FeCl 3 (s) + Н20 ---> FEHCI (aq) + ОН " (aq) D. FeCl 3 (s) ---> Fe 3+ (ад) + 3 CI (аq) ii) How many total moles of ions are released if 5.71 x1019 formula units of FeCl3 were completely dissolved? (NOTE: Write answer in sci. notation using "E" or "e" to replace "x 10" & do NOT leave any space between…arrow_forwardSuppose a 250. mL flask is filled with 0.80 mol of SO, and 1.5 mol of SO3. This reaction becomes possible: 3' 2S0,(g) +02(g) = 2SO;(g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of O,. You can leave out the M symbol for molarity. so, so, 2. initial change equilibriumarrow_forwardAn aqueous solution of 0.1 M H2SO4(aq) is added to 0.1 M Na2S(aq) and is allowed to react. From the following, select all of the statements that are true. The product of the reaction will have a colored supernatant The product of the reaction will have a colorless supernatant The reaction will produce water as a product The reaction will produce a precipitate The reaction will produce a gas The reaction will result in an oxidation and/or reduction of one of the reactantsarrow_forward
- When aqueous solutions of AgNO3 and NaCl are mixed together, the precipitate that will form is Solubility of KCl at 20 oC is 34 g/100 g H₂O. A solution containing 20 g of KCI in 50 g of water at 20 oC is classified as solution. NH3 will dissolve in water because NH3 is a solvent. solute. CCl4 is insoluble in water because water is aarrow_forwardA titration of a 0.7879g sample potassium hydrogen phthalate, KHP (GFW= 204.23g/mole), required 21.25 mL of sodium hydroxide solution to reach the end point. What is the molarity of the solution?arrow_forwardSuppose a 250. mL flask is filled with 1.3 mol of SO, and 1.6 mol of SO3. This reaction becomes possible: 250,(3) +0,(g) = 2so,(3) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of 0,. You can leave out the M symbol for molarity. so, 0, so, initial change equilibriumarrow_forward
- the number of moles of Aluminum in 1.3741 g was calculated to be 0.051 moles and that the chemistry student weighed out. For every mole of Aluminum that you start with, you can create one mole of Alum [KAl(SO4)2 ⋅12 H2O(s)]. What is the theoretical yield before drying the Alum precipitate?arrow_forwardConsider the reaction: HCI(aq) + NH, (ag) – NH,Cl(aq) Where 20.00 mL of 0.100 M NH, (aq) is added to 10.00 mL of 0.200 M HCl(aq). Order the solution components (excluding water) from highest to lowest concentration. O [OH¯] > [NH3] > [Na*] > [Cl¯] > [H,O*] O (CI ] = [NH†] > [NH,] = [H,O*]> [OH¯] O [H,0*)> [Cl¯] > [NH,] > [NH†]> [OH) O [NH]= [OH"] > [H,O*] = [CI¯] > [NH,] O [H,0*] = [CI¯] > [NH,] > [NH ] = [OH] O [NH,CI] = [NH,] = [HCl] O [CI] > [NH†]> [H,O+] > [NH,] > [OH ] O INH,CI] > [NH3] > [HCl] O None of thesearrow_forwardA solution contains one or more of the following ions: Ag+, Ca²+, and Cu²+. When sodium chloride is added to the solution, no precipitate forms. When sodium sulfate is added to the solution, a white precipitate forms. The precipitate is filtered off and sodium carbonate is added to the remaining solution, producing a precipitate.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY