
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
one reaction should be selected in each of the following four categories: (a) acid base reactions, (b) complex ion reactions, (c) gas forming reactions and (d) precipitation reactions. For each reaction write a balanced
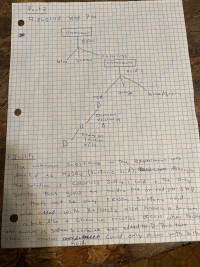
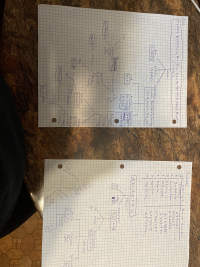
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The reaction Pb(NO 3) 2( aq) + Li 2SO 4( aq) → PbSO 4( s) + 2 LiNO 3( aq) is best classified as a(n) oxidation-reduction reaction. acid-base neutralization reaction. single replacement reaction. precipitation reaction.arrow_forwardWrite the balanced net ionic equation FeCl3(aq)+NaOH(aq)---Fe(OH)3(s)+NaCl(aq)arrow_forwardComplete and balance the following acid-base equations. (Assume that these reactions go to completion. Use the lowest possible whole number coefficients. Include states-of-matter under the given conditions in your answer.) (a) A solution of Mg(OH)2 is added to a solution of HCH3CO2. (b) A solution of HOCl reacts with solid Ni(OH)2. I am a little confused by these equations if you wouldn't mind going into detailarrow_forward
- Predict the products of each precipitation reaction, listing the solid precipitate first, and then balance the completed equation. (Enter your answers using the format CH4 for CH. Use the lowest possible coefficients.) (s)+[ (a) (aq) (b) Ca(NO3)2(aq) + Na,C,04(aq) → Pb(NO3)2(aq) + KBr(aq) → ](s) + C (aq)arrow_forwardThe following reaction occurs when two aqueous solutions are mixed: Cr(NO3)3(aq) + 3NaOH(aq) → Cr(OH)3(s) + 2NaNO3(aq) Identify the spectator ion OR ions in the solution. SELECT ALL SPECTATOR IONS!arrow_forwardPredicting the reactants of a neutralization reaction Predict the reactants of this chemical reaction. That is, fill in the left side of the chemical equation. Be sure the equation you submit is balanced. (You can edit both sides of the equation to balance it, if you need to.) Note: you are writing the molecular, and not the net ionic equation. [] → NaCIO_(aq) + H,O) H₂O(1) X 1/5 Ś ? oll 18 Ar A. For efloorarrow_forward
- write the net ionic equation for the reaction in the photoarrow_forwardA student Investigated the stoichiometry of the reaction of zinc (Zn) and HCl solution and reported the following data. When 0.2158 g of Zn reacted with 10.00 ml of 1.000M HCl, 82.062 g of water was displaced. A total of 36.00 ml of 9.501 x 10-2M NaOH solution was required to titrate the HCl remaining in the reaction mixture at the end of the reaction. The room temperature was 27.0°C and the barometric pressure was 777 torr. Calculate the volume of the displaced water from its mass (which will be equal to the volume of the gas collected.) 2) Calculate the pressure of the H2 collected 3) Calculate the number of moles of H2 collected using the ideal gas equation. 4)calculate the number of moles of hcl remaining in the flask at the end of the reaction 5) caluxukage the number of miles of hcl originally present in the reaction 6) calculate the number of moles hcl reacting 7) ccalculate the number of moles zn reacting 8) calculate the ratio of the number of hcl reacting to the number of…arrow_forwardThe concentration of SO42– ions in a 45.0 mL sample of seawater is determined by adding a solution of BaCl2 and precipitating the SO42– as BaSO4. After the precipitate is filtered from the solution, it is dried and weighed. If the mass of BaSO4 recovered is 0.315 g, what is the sulfate concentration of the seawater sample? Express your answer in mmol/L.arrow_forward
- Sodium hydrogen carbonate (NaHCO3), also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid (HC1), which the stomach secretes to help digest food. Drinking a glass of water containing dissolved NaHCO3 neutralizes excess HC1 through this reaction: HCl(aq) + NaHCO3(aq) NaCl(aq) + H₂O(1) + CO₂(g) The CO₂ gas produced is what makes you burp after drinking the solution. Suppose the fluid in the stomach of a woman suffering from indigestion can be considered to be 150. mL of a 0.010 M HCl solution. What mass of NaHCO3 would she need to ingest to neutralize this much HCl ? Round your answer to 2 significant digits. ■ x10 ×arrow_forward1. (а) The solubility product of a sparingly soluble salt, XF2, was determined by titration and found to be 2.9 x 101º M. The procedure involved the following steps: (i) A saturated solution of the salt XF2 was prepared. (ii) 25.00 mL of this solution was titrated against a standardized 0.005 M HCl solution. Determine the titre value obtained in the experiment.arrow_forwardWrite the net ionic equation for the following molecular equation. HBr is a strong electrolyte. CH3 COOH is a weak electrolyte. NaCH,COO(a) + нBr(aq) —+ NaBr(aq) + CH,COОҢ(аq) (Use the lowest possible coefficients. Be sure to specify states such as (ag) or (s). If a box is not needed, leave it blank.) +arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY