
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
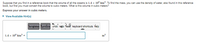
Transcribed Image Text:Suppose that you find in a reference book that the volume of all the oceans is 1.4 x 10° km. To find the mass, you can use the density of water, also found in this reference
book, but first you must convert the volume to cubic meters. What is this volume in cubic meters?
Express your answer in cubic meters.
View Available Hint(s)
Temglates Symbols undo redo Teset keyboard shortcuts Help
1.4 x 10° km
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The density of water at 4.00°C is 0.967 g/mL. How many molecules of water are present in a 499.8 mL bottle of water? Express your answer to the correct number of significant figures. Be sure to show all steps completed to arrive at the answer.arrow_forwardplease see attached imagearrow_forwardA major component of gasoline is octane (C8H18). When liquid octane is burned in air it reacts with oxygen (O₂) gas to produce carbon dioxide gas and water vapor. Calculate the moles of oxygen needed to produce 2.00 mol of carbon dioxide. Be sure your answer has a unit symbol, if necessary, and round it to 3 significant digits. 0 00 X x10 Śarrow_forward
- 3 A general chemistry student found a chunk of metal in the basement of a friend's house. To figure out what it was, she used the ideas just developed in class about density. First she measured the mass of the metal to be 183.3 grams. Then she dropped the metal into a measuring cup and found that it displaced 16.0 mL of water. Calculate the density of the metal. Density = g/mL Use the table below to decide the identity of the metal. This metal is most likely Densities of Some Common Substances Substance Density (g/mL) Water 1.00 Aluminum 2.72 Chromium 7.25 8.91 8.94 10.50 11.34 13.60 19.28 19.38 Nickel Copper Silver Lead Mercury Gold Tungsten Previous Next>arrow_forwardYou are traveling to the UK, how many US dollars are required to get £ 5.00? (£1=$1.21) step by step example pleasearrow_forwardneed help with this chemistryarrow_forward
- You are given the masses, in grams (g), and the volumes, in cubic centimeters (cm°), for a series of substances. Arrange them in order of increasing density. Rank from lowest density to highest density. To rank items as equivalent, overlap them. View Available Hint(s) Reset Help Chloroform Ice Gold 19.3 g 1 cm3 Butane Copper 44.5 g 5 cm3 NaCl 14.8 g 10 cm 92 g 100 cm3 2895 g 43 g 20 cm3 5000 cm3 Highest density Lowest densityarrow_forwardLiquid hexane CH3CH24CH3 will react with gaseous oxygen O2 to produce gaseous carbon dioxide CO2 and gaseous water H2O . Suppose 2.6 g of hexane is mixed with 13.3 g of oxygen. Calculate the minimum mass of hexane that could be left over by the chemical reaction. Be sure your answer has the correct number of significant digits.arrow_forwardCarbon disulfide gas and oxygen gas react to form sulfur dioxide gas and carbon dioxide gas. What volume of carbon dioxide would be produced by this reaction if 8.85 mL of carbon disulfide were consumed? Also, be sure your answer has a unit symbol, and is rounded to 3 significant digits. Ú 0.0 X S 0 x10arrow_forward
- please put the right number of significant figuresarrow_forwardA chemist prepares a solution of sodium chloride (NaCl) by measuring out 4.66 g of sodium chloride into a 50. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's sodium chloride solution. Round your answer to 2 significant digits. ||molL x10 Explanation Check O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Accessibility 80 F Cloudy DELL Delete PgUp PrtScr Insert F11 F12 F9 F10 F7 F8 F5 F6 Nus F3 F4 Esc F1 F2 Backspace & $arrow_forwardA chemistry student needs 85.0 g of carbon tetrachloride for an experiment. By consulting the CRC Handbook of Chemistry and Physics, the student discovers -3 Calculate the volume of carbon tetrachloride the student should pour out. that the density of carbon tetrachloride is 1.59 g cm Round your answer to 3 significant digits. mL x10 I Don't Know Submit Privacy Terms of Use © 2020 McGraw-Hill Education. All Rights Reserved. Ctv 10 MacBook Pro & 4 Y Q H. option command command tion 00 LLarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY