
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
please put the right number of significant figures
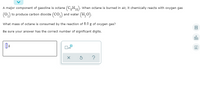
Transcribed Image Text:A major component of gasoline is octane (C,H,). When octane is burned in air, it chemically reacts with oxygen gas
18
(0,) to produce carbon dioxide (CO,) and water (H,O).
What mass of octane is consumed by the reaction of 8.0 g of oxygen gas?
Be sure your answer has the correct number of significant digits.
olo
Ar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (2×10^−5)(5.09×10^4) (7.70×10^−8) Above is listed as a fraction Express your answer using the correct number of significant figures.arrow_forwardWhat is the appropriate number of significant figures in this equation 3.5x0.251/8.02x30.0arrow_forwardCan you please fix this answer to be in decimal form in the correct amount of significant figures? Please and thank you!arrow_forward
- When doing significant figures when adding subtracting, multiple how do I know how many decimals places to move in my answer and how many significant figures should be in my answer?arrow_forwardfor this question, what would the correct number of significant figures be?arrow_forwardHow many moles of water were lost if the amount of water lost was 0.378 grams? Do not include units and assume three significant figures in all numbers. Be sure to include the zero before the decimal if the number is less than one.arrow_forward
- could you explain significant figuresarrow_forwardHorse height is measured in hands, one hand equaling exactly 4 inches. If a horse is 15.5 hands tall, what is its height in centimeters? Remember to round to the correct number of significant figures 1 inch = 2.54 cmarrow_forwardThe uncertainty in the measurement 0.0035 mg isarrow_forward
- Correct significant figures 400 x 185arrow_forwardAmmonia chemically reacts with oxygen gas to produce nitric oxide and water. What mass of ammonia is consumed by the reaction of 9.58g of oxygen gas? Be sure your answer has the correct number of significant digits.arrow_forwardthe first 2 need 1 more significant figurearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY