
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
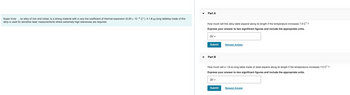
Transcribed Image Text:Super Invar, an alloy of iron and nickel, is a strong material with a very low coefficient of thermal expansion (0.20 x 10-6 C°). A 1.8-m-long tabletop made of this
alloy is used for sensitive laser measurements where extremely high tolerances are required.
Part A
How much will this alloy table expand along its length if the temperature increases 7.0 C°?
Express your answer to two significant figures and include the appropriate units.
Al=
Submit
Part B
Request Answer
How much will a 1.8-m-long table made of steel expand along its length if the temperature increases 7.0 C°?
Express your answer to two significant figures and include the appropriate units.
Al=
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- In 1986, a gargantuan iceberg broke away from the Ross Ice Shelf in Antarctica. It was approximately a rectangle 160 km long, 40.0 km wide, and 250m thick. a. What is the mass of this iceberg, in kilograms, given that the density of ice is 917 kg/m3 b. How much heat, in joules, must be transferred to the iceberg to melt it? Assume the ice is at 0° C, and the latent heat of fusion for water is Lf=79.8 kcal/kg c. How long, in years, would it take sunlight alone to melt ice this thick, if the ice absorbs an average of 100 W/m2, 12.00 hours per day?arrow_forwardA 689.00g block of gold ( cgold=129 J kg°C ) initially at 15.00°C is placed in thermal contact with an unknown mass of aluminum ( calum=900 J kg°C ) initially at 90.00°C . If they equilibrate at a final temperature of 68.00°C , what is the mass of the aluminum? g What assumption do we make when solving calorimetry problems like this? We can only have two substances in thermal contact. The substances are in a closed, isolated container that does not allow any energy to enter or leave the system. The two substances must have different specific heats. Some of the energy transferred from one substance is lost to its surroundings and does not go to the other substance.arrow_forwardFor a planet to have an atmosphere, gravity must be sufficient to keep the gas from escaping The escape speed a particle needs to escape the earth's gravitational attraction is 1.1 x 10 m/s. The motion of projectiles never depends on mass, so this escape speed applies equally to rockets and to molecules in the earth's upper atmosphere. Part A At what temperature does the rms speed of nitrogen molecules equal the escape speed? Express your answer in kelvins. ΜΕ ΑΣΦΑ T- Submit Part B T- Request An At what temperature does the mms speed of hydrogen molecules equal the escape speed? Express your answer in kelvins. VAZO Submit ? K Karrow_forward
- When a 270-g piece of iron at 160 °C is placed in a 95-g aluminum calorimeter cup containing 250 g of liquid at 10° C, the final temperature is observed to be 35 °C. The value of specific heat for iron is 450 J/kg·C°, and for aluminum is 900 J/kg · C°. Part A Determine the specific heat of the liquid. Express your answer using two significant figures. VO AE ? J C = kg-C° Submit Request Answerarrow_forwardPart C Some hydrogen gas is enclosed within a chamber being held at 200°C with a volume of 0.0250 m³. The chamber is fitted with a movable piston. Initially, the pressure in the gas is 1.50 × 106 Pa (14.8 atm). The piston is slowly extracted until the pressure in the gas falls to 0.950 x 106 Pa. What is the final volume V₂ of the container? Assume that no gas escapes and that the temperature remains at 200°C. Enter your answer numerically in cubic meters. ► View Available Hint(s) V₂ = Submit [5] ΑΣΦ ? m³arrow_forwardThe height of the Eiffel tower is 324 m, and let's assume it's made completely of steel, which has a thermal coefficient of 1.20 × 10-5/°C. a. How much taller, in meters, does the Eiffel Tower become at the end of a day when the temperature has increased by 17.5°C?arrow_forward
- In this problem you will consider the effect that thermal expansion due to temperature will have on Archimedes' principle. Take the densities of water and copper at 0ºC to be 1.00 × 103 kg/m3 and 8.90 × 103 kg/m3, respectively. a. Calculate the fraction of a copper block’s weight that is supported by the buoyant force at 0°C. b. Calculate the fraction of a copper block’s weight that is supported by the buoyant force at 95°C. Assume the volume expansion coefficient of copper is βC = 5.10 × 10-5 1/°C.arrow_forwardAluminum rivets used in airplane construction are made slightly larger than the rivet holes and cooled by "dry ice" (solid CO₂) before being driven. Part A If the diameter of a hole is 4.500 mm, what should be the diameter of a rivet at 23.0°C, if its diameter is to equal that of the hole when the rivet is cooled to -78.0°C, the temperature of dry ice? Assume that the expansion coefficient of aluminum is 2.4 x 10-5 (°C)-¹ and remains constant. -1 Express your answer in milimeters to four significant figures. d = Submit 15. ΑΣΦ Request Answer ? Review Constants mmarrow_forwardA 40-cm-long icicle hangs from the eave of a house on a day when the temperature is -2.0°C. T Part A By how many millimeters does the icicle shrink if a bitterly cold wind drops the temperature to -20 °C? Express your answer with the appropriate units. AL= Submit xa μA Value 77 X.10n Units Previous Answers Request Answer ?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON