
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
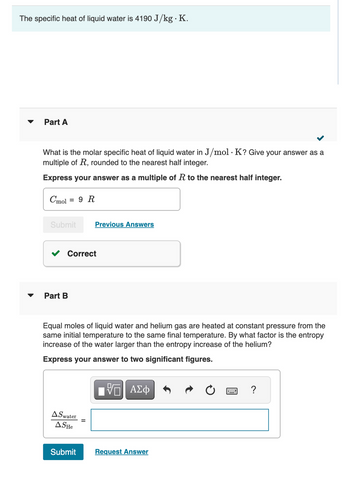
Transcribed Image Text:The specific heat of liquid water is 4190 J/kg. K.
▼
Part A
What is the molar specific heat of liquid water in J/mol. K? Give your answer as a
multiple of R, rounded to the nearest half integer.
Express your answer as a multiple of R to the nearest half integer.
Cmol = 9 R
Submit
Correct
Part B
Previous Answers
Equal moles of liquid water and helium gas are heated at constant pressure from the
same initial temperature to the same final temperature. By what factor is the entropy
increase of the water larger than the entropy increase of the helium?
Express your answer to two significant figures.
AS water
ASHe
Submit
17| ΑΣΦ
Request Answer
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 5.0 g of nitrogen gas at 20°C and an initial pressure of 2.8 atm undergo a constant-pressure expansion until the volume has tripled. Part A What is the gas volume after the expansion? Express your answer with the appropriate units. ► View Available Hint(s) V= Submit HA Value Previous Answers Units ? X Incorrect; Try Again; 5 attempts remainingarrow_forwardA diving bell is a 5.0 m-tall cylinder closed at the upper end but open at the lower end. The temperature of the air in the bell is 30 °C. The bell is lowered into the ocean until its lower end is 100 m deep. The temperature at that depth is 10°C. Part A How high does the water rise in the bell after enough time has passed for the air to reach thermal equilibrium? Express your answer with the appropriate units. h = Submit Part B μA Value Request Answer Units ? A compressed-air hose from the surface is used to expel all the water from the bell. What minimum air pressure is needed to do this? Express your answer with the appropriate units.arrow_forwardA cylinder contains 0.250 mol of carbon dioxide (CO₂) gas at a temperature of 27.0°C. The cylinder is provided with a frictionless piston, which maintains a constant pressure of 1.00 atm on the gas. The gas is heated until its temperature increases to 127.0°C. Assume that the CO₂ may be treated as an ideal gas. Part A How much work is done by the gas in this process? Express your answer in joules. — ΑΣΦ W = Submit Part B On what is this work done? The work is done on the piston. The work is done on the cylinder. Submit Part C Request Answ AU = What is the change in internal energy of the gas? Express your answer in joules. ΑΣΦ Submit Request Answer Request Answer P Pearson W ? ? J Jarrow_forward
- A cylinder is filled with 10.0L of gas and a piston is put into it. The initial pressureof gas is measured to be 255. kPa. The piston is now pushed down, compressingnthe gas, until the gas has a final volume of 4.70 L calcuate the final pressure of the gas. Round your answer to 3 significant digitsarrow_forwardIn a container of negligible mass, 0.300 kg of ice at an initial temperature of -25.0°C is mixed with a mass m of water that has an initial temperature of 80.0°C. No heat is lost to the surroundings. Part A If the final temperature of the system is 19.0°C, what is the mass m of the water that was initially at 80.0°C? Express your answer with the appropriate units. m = Submit μA Value Request Answer Units ? Reviewarrow_forwardPart A The value of specific heat for copper is 390 J/kg Co, for aluminun is 900 J/kg. C°, and for water is 4186 J/kg. Co. What will be the equilibrium temperature when a 235 g block of copper at 235 °C is placed in a 145 g aluminum calorimeter cup containing 865 g of water at 14.0°C? Express your answer using three significant figures. T = ΜΕ ΑΣΦ 0 ? °Carrow_forward
- 5.0 g of nitrogen gas at 20°C and an initial pressure of 2.8 atm undergo a constant-pressure expansion until the volume has tripled. Part C How much heat is transferred to the gas to cause this expansion? Express your answer with the appropriate units. ► View Available Hint(s) Qexp 3000 J Submit Previous Answers ✓ Correct The gas pressure is then decreased at constant volume until the original temperature is reached. Part D What is the gas pressure after the decrease? Express your answer with the appropriate units. View Available Hint(s)arrow_forwardAn ideal gas at 20°C consists of 2.2 x 1022 atoms. 3.2 J of thermal energy are removed from the gas. Part A What is the new temperature in °C? Express your answer in degrees Celsius. DA ΑΣφ T = °C Submit Request Answerarrow_forwardT5 please help me with the answer and full solutionarrow_forward
- Perfectly rigid containers each hold n moles of ideal gas, one being hydrogen (H₂) and other being neon (Ne). Part A If it takes 300 J of heat to increase the temperature of the hydrogen by 2.70°C, by how many degrees will the same amount of heat raise the temperature of the neon? Express your answer in degrees Celsius. ATNe= Submit 17 ΑΣΦ Request Answer ? °Carrow_forwardA cylinder contains 0.250 mol of carbon dioxide (CO₂) gas at a temperature of 27.0°C. The cylinder is provided with a frictionless piston, which maintains a constant pressure of 1.00 atm on the gas. The gas is heated until its temperature increases to 127.0°C. Assume that the CO₂ may be treated as an ideal gas. Express your answer in joules. AU = Submit Part D Submit How much heat was supplied to the gas? Express your answer in joules. Part E W₁ = ΑΣΦ | Submit Request Answer ΑΣΦ Request Answer Request Answer < Return to Assignment Provide Feedback www How much work would have been done if the pressure had been 0.50 atm? Express your answer in joules. VE ΑΣΦ P Pearson = ? ? J ? J Jarrow_forwardThe basal metabolic rate is the rate at which energy is produced in the body when a person is at rest. A 68 kg (150 lb) person of height 1.83 m (6.0 ft) would have a body surface area of approximately 1.9 m². Part A What is the net amount of heat this person could radiate per second into a room at 19.0° C (about 66.2°F) if his skin's surface temperature is 31.0° C? (At such temperatures, nearly all the heat is infrared radiation, for which the body's emissivity is 1.0, regardless of the amount of pigment.) Express your answer in watts. ΑΣφ ? Hnet W %D Submit Request Answer Part B Complete previous part(s)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON