
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN: 9781305580343
Author: Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
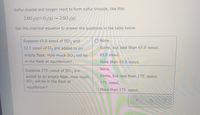
Transcribed Image Text:Sulfur dioxide and oxygen react to form sulfur trioxide, like this:
2 SO 2(g)+O2(9) –
2 SO 3(9)
Use this chemical equation to answer the questions in the table below.
Suppose 65.0 mmol of SO, and
None.
32.5 mmol of O, are added to an
Some, but less than 65.0 mmol.
empty flask. How much SO,3 will be
65.0 mmol.
in the flask at equilibrium?
More than 65.0 mmol.
Suppose 175. mmol of SO, are
O None.
added to
empty flask. How much
Some, but less than 175. mmol.
SO, will be in the flask at
O 175. mmol.
equilibrium?
O More than 175. mmol.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Carbon dioxide from the atmosphere weathers, or dissolves, limestone (CaCO3) by the reaction CaCO3(s)+CO2(g)+H2O(l)Ca2(aq)+2HCO3(aq) Obtain H for this reaction. See Table 6.2 for the data.arrow_forward4.90 Iron metal can be refined (rom the mineral hematite (Fe2O3). One way of converting the mineral to iron is to react it with carbon monoxide, as shown below: Fe2O3+3CO2Fe+3CO2 Because the hematite is obtained from various ores, it is usually not in a pure form. Suppose an iron manufacturer has 2.00 X 105 kg of ore available, and the ore is 93% Fe2O3 by mass. (There is no iron in the remaining 7% of the ore.) How many moles of Fe2O3are present in this ore? How many kg of pure iron could be obtained from this sample of ore? Assume that the process has a 100% yield and that excess CO is available.arrow_forwardThe carbon dioxide exhaled in the breath of astronauts is often removed from the spacecraft by reaction with lithium hydroxide 2LiOH(s)+CO2(g)Li2CO3(s)+H2O(l) Estimate the grams of lithium hydroxide required per astronaut per day. Assume that each astronaut requires 2.50 103 kcal of energy per day. Further assume that this energy can be equated to the heat of combustion of a quantity of glucose, C6H12O6, to CO2(g) and H2O(l). From the amount of glucose required to give 2.50 103 kcal of heat, calculate the amount of CO2 produced and hence the amount of LiOH required. The H for glucose(s) is 1273 kJ/mol.arrow_forward
- The pictures below show a molecular-scale view of a chemical reaction between H2 and CO to produce methanol, CH3OH The box on the left represents the reactants at the instant of mixing, and the box on the right shows what is left once the reaction has gone to completion. D Was there a limiting reactant in this reaction? If so, what was it? Write a balanced chemical equation for this reaction. As usual, your equation should use the smallest possible whole number coefficients for all substances.arrow_forward4.69 The pictures below show a molecular-scale view of a chemical reaction between H2 and CO to produce methanol, CH3OH. The box on the left represents the reactants at the instant of mixing, and the box on the right shows what is left once the reaction has gone to completion. Was there a limiting reactant in this reaction? If so, what was it? Write a balanced chemical equation for this reaction. As usual, your equation should use the smallest possible whole number coefficients for all substances.arrow_forward4.21 Ammonium nitrate, NH4NO3, will decompose explosively to form N2, O2, and H2O, a fact that has been exploited in terrorist bombings. What mass of nitrogen is formed by the decomposition of 2.6 kg of ammonium nitrate?arrow_forward
- Write a balanced equation for the reaction between (a) dihydrogen sulfide and sulfur dioxide gases to form sulfur solid and steam. (b) methane, ammonia, and oxygen gases to form hydrogen cyanide gas and steam. (c) iron(lll) oxide and hydrogen gas to form molten iron and steam. (d) uranium(IV) oxide and hydrogen fluoride gas to form uranium(IV) fluoride and steam. (e) the combustion of ethyl alcohol (C2H5OH) to give carbon dioxide and water.arrow_forwardA 100.0-g mixture made up of NaCl03, Na2CO3, NaCl, and NaHCO3 is heated, producing 5.95 g of oxygen, 1.67 g of water, and 14.5 g of carbon dioxide. NaCl does not react under the conditions of the experiment. The equations for the reactions that take place are: 2NaClO3(s)2NaCl(s)+3O2(g)Na2CO3(s)2Na2O(s)+CO2(g)2NaHCO3(s)Na2O(s)+2CO2(g)+H2O Assuming 100% decomposition of NaClO3, Na2CO3, and NaHCO3, what is the composition of the mixture in grams?arrow_forwardSmall quantities of oxygen gas can be generated in the laboratory by the decomposition of hydrogen peroxide. The unbalanced equation for the reaction is H2O2(uz/)-? H2O(/) + O2(g) Calculate the mass of oxygen produced when 10.00 g of hydrogen peroxide decomposes.arrow_forward
- 4-77 To convert 1 mol of iron(III) oxide to its elements requires 196.5 kcal: How many grams of iron can be produced if 156.0 kcal of heat is absorbed by a large-enough sample of iron(III) oxide?arrow_forwardEthanol, C2H5OH, is a gasoline additive that can be produced by fermentation of glucose. C6H12O62C2H5OH+2CO2 (a) Calculate the mass (g) of ethanol produced by the fermentation of 1.000 lb glucose. (b) Gasohol is a mixture of 10.00 mL ethanol per 90.00 mL gasoline. Calculate the mass (in g) of glucose required to produce the ethanol in 1.00 gal gasohol. Density of ethanol = 0.785 g/mL. (c) By 2022, the U. S. Energy Independence and Security Act calls for annual production of 3.6 1010 gal of ethanol, no more than 40% of it produced by fermentation of corn. Fermentation of 1 ton (2.2 103 lb) of corn yields approximately 106 gal of ethanol. The average corn yield in the United States is about 2.1 105 lb per 1.0 105 m2. Calculate the acreage (in m2) required to raise corn solely for ethanol production in 2022 in the United States.arrow_forwardFor this reaction, fill in the table with the indicated quantities for the balanced equation. 4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(g)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning