
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%

Transcribed Image Text:Suggest reagents and experimental conditions for each step in this synthesis.
From the choices provided, suggest appropriate reagents for each step. More than one reagent may be necessary. Use the minimum number of steps
possible. Enter your answer as a letter, or a series of letters, in the order necessary to bring about the steps shown.
Reagents:
(1)
(2)
(3)
(4)
(a) CH3O Na+, CH3OH (d) H₂O
(b) OsO4, H₂O2
(c) 1. BH3
2. H₂O2/NaOH
(e) NaOH, H₂O
(f) CH3 CH₂ OH
(9) CH3 CN
(j) Na, NH3 (1)
(h) Naº, THF (k) NBS, light
(1) PBr3
(N-bromosuccinimide)
(l) NaNH,
Previous
Next
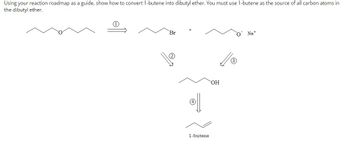
Transcribed Image Text:Using your reaction roadmap as a guide, show how to convert 1-butene into dibutyl ether. You must use 1-butene as the source of all carbon atoms in
the dibutyl ether.
Br
1-butene
OH
O Na+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using Ethylene, C2H4, and methyl bromide, CH3Br, as your sole sources of carbon, propose a synthesis for t-BuOH, (CH3)3COH. *the synthesis should take about 7 steps and will include an alkylation and a Grignard reaction. Use one equivalent of methyl bromide for the alkylation step and use the second one to make the Grignard reagent. The substrate in the Grignard reaction will be a carbonyl - make this from your alkyne AFTER alkylation. *show all reactants and intermediates H +2 H H Br -7 steps H₂C H₂C OH CH3arrow_forward7) Using the carbon-containing starting material(s), propose a synthesis the following retrosynthetic analysis. Provide structures for all intermediates. The carbon atoms in the product must originate from the starting material(s), but you may use as many equivalents of each starting material as you would like, and any reagent/reaction you know (note: no mechanisms are required). Br OH racemic = = + -Brarrow_forwardGive the reagents for the following reaction.arrow_forward
- HA,BH,HB all incorrectarrow_forwardUsing any necessary reagents, show how the following synthetic transformations may be achieved. Give reagents and conditions for each step. Include all synthetic intermediates (compounds produced during the course of multi-step synthesis). NOTE: You may not use HO CI OH NH₂arrow_forwardIdentify the reagents you would use to perform the following transformation: Bromocyclohexane →→ Cyclohexanecarboxylic acid The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagents in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A. H3O+, heat D. 1) CO2; 2) H3O+ B. Na₂Cr₂O7, H₂SO4, H₂O E. PCC or DMP C. NaCN F. Mgarrow_forward
- Identify the reagents you would use to convert 1-bromopentane into hexanoic acid. 1-bromopentane → hexanoic acid The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagents in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A. PCC or DMP D. H3O+, heat G. Na₂Cr₂O7, H₂SO4, H₂O B. NaCN E. Mg H. 1) LiAlH4; 2) H3O+ C. [H*], EtOH F. NaOH I. 1) CO₂; 2) H3O+arrow_forwardProvide the major product for the following reaction?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY