
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
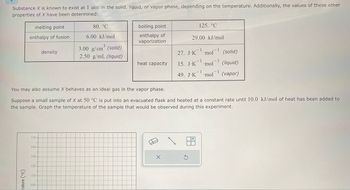
Transcribed Image Text:Substance X is known to exist at 1 atm in the solid, liquid, or vapor phase, depending on the temperature. Additionally, the values of these other
properties of X have been determined:
melting point
enthalpy of fusion
rature (°C)
150.
140-
130.
120-
110-
density
100-
80. °C
6.00 kJ/mol
3.00 g/cm³ (solid)
2.50 g/mL (liquid)
boiling point
enthalpy of
vaporization
You may also assume X behaves as an ideal gas in the vapor phase.
Suppose a small sample of X at 50 °C is put into an evacuated flask and heated at a constant rate until 10.0 kJ/mol of heat has been added to
the sample. Graph the temperature of the sample that would be observed during this experiment.
heat capacity
125. °C
X
29.00 kJ/mol
1
27. J.K mol
15. J.K 'mol
-1
49. J.K mol
-1
(solid)
(liquid)
(vapor)
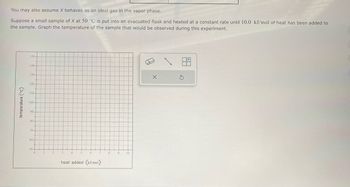
Transcribed Image Text:You may also assume X behaves as an ideal gas in the vapor phase.
Suppose a small sample of X at 50 °C is put into an evacuated flask and heated at a constant rate until 10.0 kJ/mol of heat has been added to
the sample. Graph the temperature of the sample that would be observed during this experiment.
temperature (°C)
150-
140-
130-
120-
110-
100
90
80-
70-
60
50
0
heat added (kJ/mol)
10.
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The normal boiling point of a liquid is 282 °C. At what temperature (in °C) would the vapor pressure be 0.230 atm? (AHvap = 28.5 kJ/mol) %3D 20arrow_forwardHow much heat energy is required to convert 39.6 g of solid ethanol at -114.5 °C to gaseous ethanol at 147.2 °C? The molar heat of fusion of ethanol is 4.60 kJ/mol, and its molar heat of vaporization is 38.56 kJ/mol. Ethanol has a normal melting point of −114.5 °C and a normal boiling point of 78.4 °C. The specific heat capacity of liquid ethanol is 2.45 J/g. °C, and that of gaseous ethanol is 1.43 J/g. °C. q= 4326.84 kJarrow_forwardSubstance X is known to exist at 1 atm in the solid, liquid, or vapor phase, depending on the temperature. Additionally, the values of these other properties of X have been determined: melting point 30. °C boiling point 90. °C enthalpy of vaporization enthalpy of fusion 8.00 kJ/mol 29.00 kJ/mol 1.70 g/cm (solid) 1.50 g/mL (liquid) density 35. J·K 1- •mol (solid) heat capacity 45. J'K (liquid) "mol 55. J·K •mol (vapor) You may also assume X behaves as an ideal gas in the vapor phase. Suppose a small sample of X at 0 °C is put into an evacuated flask and heated at a constant rate until 15.0 kJ/mol of heat has been added to the sample. Graph the temperature of the sample that would be observed during this experiment.arrow_forward
- Use the phase diagram of Substance X below to find the pressure at which the melting point of X is - 173. °C. 1.6- solid liquid 0.8- gas 100 200 temperature (K) Note: your answer must be within 0.1 atm of the exact answer to be graded correct. atm pressure (atm)arrow_forwardpls help asap! make sure the graph is correct and cleararrow_forward1. Calculate the amount of heat (in kJ) released when converting 150.2 g of water to ice at -23 degrees Celsius. 2. A closed vessel of volume 10.0 L initially contains 1.303 g of water and no water vapor. Please calculate the mass of liquid water remaining once the system reaches equilibrium at 30 degrees Celsius. The vapor pressure of water at that temperature is equal to 0.0418 atm. 3. A person drinks four glasses of cold water (5 degrees C) every day. The volume of each glass is 195. mL. How much heat (in kJ) does the body have to supply to raise the temperature of the water to 37 degrees C (body temperature)? 4.From these data: N2H4(l) + O2(g) à 2N2(g) + 2H2O(l) H= -622.2 kJ/mol H2(g) + ½O2 à H2O(l) H= -285.8 kJ/mol H2(g) + O2(g) à H2O2(l) H= -115 kJ/mol Find H for the reaction: N2H4(l) + 2H2O2(l) à 2N2(g) + 4H2O(l) 5. Consider the following chemical equation. CH4(g) + 2O2(g) à CO2(g) + 2H2O(l) Using the values in the table, calculate…arrow_forward
- Substance X is known to exist at 1 atm in the solid, liquid, or vapor phase, depending on the temperature. Additionally, the values of these other properties of X have been determined: melting point enthalpy of fusion - 10. °C boiling point 4.00 kJ/mol enthalpy of vaporization 30. °C 39.00 kJ/mol 3 1.60 g/cm³ (solid) 1 1 density 44. J.K ·mol (solid) 1.30 g/mL (liquid) 1 -1 heat capacity 64. J.K ·mol (liquid) 1 1 54. J.K mol (vapor) You may also assume X behaves as an ideal gas in the vapor phase. Suppose a small sample of X at -50 °C is put into an evacuated flask and heated at a constant rate until 10.0 kJ/mol of heat has been added to the sample. Graph the temperature of the sample that would be observed during this experiment. temperature (°C) 50- 40 30. 20 10. -10 -20 -30 -40 -50 0 1 2 3 4 5 6 7 8 9 10 heat added (kJ/mol) ☑ ? 00. 18 Ararrow_forwardREFER TO IMAGEarrow_forwardPrior to the discovery that freon-12 (CF2Cl2) was harmful to the Earth's ozone layer, it was frequently used as the dispersing agent in spray cans for hair spray, etc. Its enthalpy of vaporization at its normal boiling point of -29.2 degree C is 20.25 kJ/mol. Estimate the pressure that a can of hair spray using freon-12 had to withstand at 40 degree C, the temperature of a can that has standing in sunlight.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY