
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:N MyLab and Mastering
Course Home
b Search results for "Which of the
At Electronegativity Table of the Ele x
+
A openvellum.ecollege.com/course.html?courseld=16418591&OpenVellumHMAC=fd2ce09746a2194453c324a109d1a8ec#10001
Scores
Part A
Pearson eText
Part B
Study Area
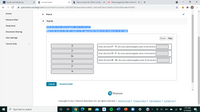
Indicate the more electronegative atom in each pair.
Match the atoms in the left column to the appropriate blanks in the sentences on the right.
Document Sharing
User Settings
Reset Help
Course Tools
>
B
Given the bond P-N, the more electronegative atom in the bond is
Given the bond B-F, the more electronegative atom in the bond is
Br
Given the bond H-Br, the more electronegative atom in the bond is
H
P
N
Submit
Request Answer
P Pearson
Copyright © 2021 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy. | Permissions | Contact Us |
2:55 AM
P Type here to search
4/14/2021
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2RO; (aq) + 12 H* (aq) + 3 M (s) = RO (g)+3M²* (aq) + 6 H2O (1) = 3.86 V. R: 5 valence e-, electronegativity of R = 3.3 M: 2 valence e-, electronegativity of M = 0.5 O and H represent Oxygen and Hydrogen (the known elements, with their known properties) All atoms follow the octet rule; except for Hydrogen which follows the duet rule. b) Calculate the new cell potential if concentrations of all the reactants is deceased to 0.5 M, and the concentrations of all the products is increased to 0.5 M. Assume 6 mol electrons transferred in the reaction.arrow_forwardD Lewis strct molecalar iodie, and the dide inarrow_forward2RO; (aq) + 12 H* (aq) + 3 M (s) = RO(g)+ 3M²* (aq) + 6 H2O (1) E°rxn = 3.86 V. R: 5 valence e-, electronegativity of R = 3.3 M: 2 valence e-, electronegativity of M = 0.5 O and H represent Oxygen and Hydrogen (the known elements, with their known properties) All atoms follow the octet rule; except for Hydrogen which follows the duet rule. Does the above reaction favour the forward or reverse direction? If the concentration of RO; (ag) was reduced and What is the pH of this reaction? explain the reasons for your answers.arrow_forward
- ofiles Window Help Tab Tu - Minc x A Mas x V My ( x Para X M Your x GS The x A Acti x C 1) D X C Nlc x b Chrc x Irn/takeAssignment/takeCovalentActivity.do?locator=assignment-take * O [Review Topics] [References) Use the References to access important values if needed for this question. 3 :* * : :X The Lewis representation above depicts a reaction between a halogen (blue) and a main-group element from group (red). In this representation, each Y atom needs electron(s) to complete its octet, and gains these electrons by forming bond(s) with atoms of X. There are unshared electron pair(s) and bonding electron pair(s) in the product molecule. The bonds in the product are Retry Entire Group 9 more group attempts remaining Submit Answer (Previous Next Email Instructor Save and Exi Aa étv S MAR 15 G Search or type URLarrow_forwardenow.com/ilrn/takeAssignment/takeCovalentActivity.do?locator%3Dassignment-take [References] Acetic acia is responsibie for the sour taste or vihegar. it can be manuracturea using the following reaction: |3| 2 CH3C-OH(g) 2 CH3C-H(g) + O2(g) Use tabulated values of bond energies from the table below to estimate AH for this reaction. Average Bond Energies Single Bonds Bond Bond Energy (kJ/mol) C-C 347 C-H 413 C-O 358 0-H 467 Multiple Bonds Bond Bond Energy (kJ/mol) C=0 1072 C=0 745 O=0 to 495 kJ %3Darrow_forwardeducation.wiley.com/was/ui/v2/assessment-player/index.html?launchld=06c032ef-b96c-4463-b835-41e474f31335#/question/23 How should the sulfur-oxygen bond lengths compare for the species So, SO2, So,, and so,?? 8Incorrect. Select the compound with the longest bond length. O SO3 O SO2 O so,2- O so.2 Hint Incorrect. Select the compound(s) with the second longest bond length(s). O SO3 O So2 O so,- O so, Assistance Used Hint 2 Incorrect. Select the compound with the shortest bond length. O So3 O SO2 O so,? O so,2arrow_forward
- 3. The skeleta ucture of acetic acid shown below As correct, bonds are wrong.c@ Identify the incorret bonds and exploun what is wrong them · 6 Wite the co ct Lewis cture for acetic acid. EG but some of the with :0: H =C-C -0-H A HICIHarrow_forwards please answer allarrow_forwardOnl X S Write electro x uzdLQYhAS) X G Predict the t X Periodic Tabl x b Answered: [F X mySigTau x M (no subject) X O Electronegat X enow.com/ilrn/takeAssignment/takeCovalentActivity.do?locator=Dassignment-take [References] Choose the name of each of the following molecular structures. Molecular structure Name a. C. d. > b.arrow_forward
- 21 Onl x S Write electrc X uzdLQYhASX x G Predict thet X t x Periodic Tab x b Answered: x 0mySigTau X M (no subject) > genow.com/ilrn/takeAssignment/takeCovalentActivity.do?locator=Dassignment-take [References] Consider the following Lewis structure where E is an unknown element: :ö: 0-E-O :( What is a possible identity for element E? Predict the molecular structure (including bond angles) for this ion. Molecular shape is v with bond anglesarrow_forwardN Gve the structure of the mayor produc CrO, HSO HO- OH PCC 2. CH;Ch HBr, ROOR Ph AICarrow_forwardwpu-yomx-kpa (2020-12-03 at 0 x+ pom.google.com/w/MTMxMDcyMTgzNTI2/t/all I GPA C.. O Unit Landing Page |. Classes 8) Write resonance forms that describe the distribution of electrons in each of the molecules or ions given below: (a) selenium dioxide, OSEO (b) nitrate ion, NO, (c) nitric acid, HNO, (N is bonded to an OH group and two O atoms) (d) benzene, CH,:arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning
