
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
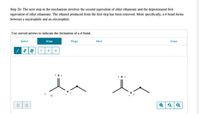
Transcribed Image Text:Step 2b: The next step in the mechanism involves the second equivalent of ethyl ethanoate and the deprotonated first
equivalent of ethyl ethanoate. The ethanol produced from the first step has been removed. More specifically, a o bond forms
between a nucleophile and an electrophile.
Use curved arrows to indicate the formation of a o bond.
Select
Draw
Rings
More
Erase
:0:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the simplest mechanism possible for the reaction below. You may need to re-draw structures to show bond lines or lone pairs. Note to advanced students: There may be more than one resonance structure for one of your products. Make sure the mechanism you draw creates the resonance structure that's shown. + но + H₂Oarrow_forwardFor the following Sy2 reaction, draw the organic and inorganic products of the reaction, and identify the nucleophile, substrate, and leaving group. Include wedge/dash bonds and H on a stereocenter N + Draw organic product Draw inorganic product Select the statement that properly identifies the nucleophile, substrate, and leaving group CN is the substrate, CI is the nucleophile, and CH3CH2CH2CH(CH3)Cl is the leaving group. Cr is the substrate, CH3CH2CH2CH(CH3)Cl is the nucleophile, and CN is the leaving group. CH3CH2CH2CH (CH3)CI is the substrate, CN is the nucleophile, and C is the leaving group.arrow_forwardFor this mechanism, how come the iodine doesn't attach to a carbocation on the right (with the two methyl groups coming off of it)? Is it because TsO is a better leaving group? If so, why is this?arrow_forward
- Hello, I do not understand this questions and I am stuck. May I get help please?? Question: Draw the curved arrow mechanism for the following reactionarrow_forwardDraw both resonance structures of the most stable carbocation intermediate in the reaction shown.arrow_forwardComplete the mechanism for the base-catalyzed opening of the epoxide by adding any missing atoms, bonds, charges, nonbonding electron pairs, and curved arrows. Step 1: add curved arrows. Step 2: complete the structure and add curved arrOwS. Select Draw Rings More Erase Select Draw Rings More Erase H. H 0arrow_forward
- How come in step 3, water doesn't attack the carbocation? Wouldn't it naturally want to immediately neutralize the carbocation instead of creating a double bond?arrow_forward3) Any compound or ion with at least one lone pair can be considered a base and/or a nucleophile. The relative basicity and nucleophilicity of the species is one of the most important factors that determine the mechanism of a reaction on sp³-carbon atoms. Please show an example of a) a reagent that is a strong nucleophile and a strong base, b) a reagent that is a weak nucleophile and a strong base, c) a reagent that is a strong nucleophile and a weak base, d) a reagent that is a weak nucleophile and a weak base. Which nitrogen atom in the structure below is most nucleophilic? Please explain by discussing the electron density around each nitrogen atom. Show at least three resonance structures of the compound. N.arrow_forwardDraw the major organic product(s) of the following reaction. CI CH3OH NaOCH3 You do not have to consider stereochemistry. • If no reaction occurs, draw the organic starting material. • When Sy1 & E1 pathways compete, show both the substitution and the elimination products. • Separate multiple products using the + sign from the drop-down menu. • Do not include counter-ions, e.g., Na", I', in your answer.arrow_forward
- Draw the organic product of the acid-base reaction between the species shown below. Two equivalents of –OH are used.arrow_forwardFor the given SN2 reaction, draw the organic and inorganic products of the reaction, and identify the nucleophile, substrate, and leaving group. Include wedge-and-dash bonds and draw hydrogen on a stereocenter. Hmm.. CEN + CI Organic product Inorganic productarrow_forwardUsing curved arrows, draw the complete stepwise mechanism for the following reaction: ← OH 0* h HBr A H-Br: Br Add/Remove step X Ś Ć Click and drag to startarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY