
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
#10
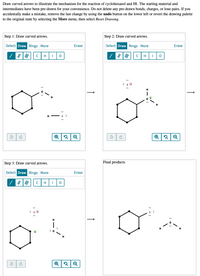
Transcribed Image Text:**Illustration of the Reaction Mechanism for Cyclohexanol with HI**
This guide provides a step-by-step illustration of the reaction mechanism of cyclohexanol with hydrogen iodide (HI). The diagrams demonstrate the use of curved arrows to indicate electron movement. The starting materials and intermediates are provided for clarity. Below is the detailed transcription and explanation for each step.
**Text Overview:**
- The reaction involves cyclohexanol and HI.
- Curved arrows are used to depict electron movement during the reaction.
- If mistakes are made, the undo button can reverse the last change, or the drawing can be reset via the More menu.
**Diagram Steps:**
**Step 1:**
- **Action:** Draw curved arrows.
- **Tools and Options:** Select, Draw, Rings, More, Erase (with options for single, double, triple, and aromatic bonds, and elements C, H, I, O).
- **Diagram Details:**
- A hexagonal cyclohexanol structure is shown with an -OH group.
- An HI molecule is present nearby.
- Electron pairs on oxygen are depicted as dots.
**Step 2:**
- **Action:** Draw curved arrows.
- **Diagram Details:**
- The HI molecule approaches the oxygen in the OH group.
- Curved arrows indicate the movement of electrons from the lone pair on oxygen to hydrogen, and from the H-I bond to the iodine atom.
- Oxygen temporarily gains a positive charge, while iodine gains a negative charge.
**Step 3:**
- **Action:** Draw curved arrows.
- **Diagram Details:**
- Highlighted intermediate stage where iodine with negative charge is separated.
- Cyclohexanol and iodide ion interact, forming the product.
**Final Products:**
- **Diagram Details:**
- The final product shows cyclohexane with an iodine atom, and the separation of water (H₂O).
These diagrams help visualize the mechanistic steps involved in the reaction, highlighting the electron movement that leads to the final products.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I need help with both questions pleasearrow_forwardA cake requires 3 eggs, 4 cups of flour, 2 cups of sugar, 0.5 cups of oil, and 1 stick of butter. In the refrigerated pantry, a chef has the following available: 56 sticks of butter 60 eggs 12 gallons (192 cups) of oil 56 cups of flour 41 cups of sugar Select the four excess reagents. Do not select the limiting reagent. butter flour eggs sugar oilarrow_forwardDo not give handwriting solution.arrow_forward
- The following arrows are incorrectly drawn and I drawn them correctly please check if it's right and write a little explanation how it's rightarrow_forward11-The branch of chemistry that is responsible for studying the elements and their compounds, excluding almost all carbon compounds ("C") is known as:Unique option. A) Biochemistry B) Analytic chemistry C) Chemistry-physics D) Inorganic chemistry E) Organic chemistryarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY