
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
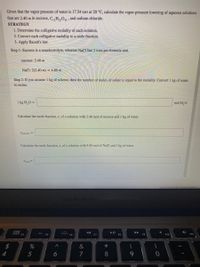
Transcribed Image Text:Given that the vapor pressure of water is 17.54 torr at 20 °C, calculate the vapor-pressure lowering of aqueous solutions
that are 2.40 m in sucrose, C,,H,01 , and sodium chloride.
STRATEGY
1. Determine the colligative molality of each solution.
2. Convert each colligative molality to a mole fraction.
3. Apply Raoult's law.
Step 1: Sucrose is a nonelectrolyte, whereas NaCl has 2 ions per formula unit.
sucrose: 2.40 m
NaCl: 2(2.40 m) = 4.80 m
Step 2: If you assume 1 kg of solvent, then the number of moles of solute is equal to the molality. Convert 1 kg of water
to moles.
1 kg H,0 =
H,O
Calculate the mole fraction, x, of a solution with 2.40 mol of sucrose and 1 kg of water.
Xsucrose =
Calculate the mole fraction, x, of a solution with 4.80 mol of NaCl and 1 kg of water.
Question Source: MRG-General Chemistry
MacBook Air
000
D00 F4
F5
F7
F8
F9
F10
&
*
4
5
6
7
8
9
Expert Solution
arrow_forward
Step 1
a) Moles of 1 kg water
1 kg = 1000 g
Moles of water = 55.56 mol
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Find the molality of this aqueous solution 15.0% by mass kBr (119g/mol).arrow_forward1. A solution is prepared by dissolving 12 grams of turmeric powder in 350 grams of water. Calculate the mass percent of each component of the solution. 2. 30 grams of ethyl alcohol (C2H$OH) is dissolved in 75 grams of water. Calculate the mole fraction of ethyl alcohol and water in the solution. 3. A solution is prepared by dissolving 51.30 g of NH,Cl (ammonium chloride) into enough water to make 600 mL of solution. Calculate its molarity. 4. Calculate the molality of a solution prepared from 30.25 grams of NaOH (sodium hydroxide) in 5.00 kilograms of water. 5. If a beaker contains 2.0 moles of water. How many grams of water are the beaker?arrow_forwardA 0.5 M NaOH solution has a density of 1.0295 g/mL. Calculate the w% and the molality of the 0.5 M NaOH solution.arrow_forward
- A pure solvent has a freezing point of −3.62°C. A solute is added to the solvent and the solution freezes at −7.81°C. If the solute is RbF what is the molality of the solution. kf,solvent = 3.68°C•kg/molarrow_forwardA solution was prepared by dissolving 200.0 g of KClKCl in 285 g of water. Calculate the mole fraction of KClKCl. (The formula weight of KClKCl is 74.6 g/molg/mol. The formula weight of water is 18.0 g/molg/mol.) Express the mole fraction of KCl to two decimal places.arrow_forward245. mg of an unknown protein are dissolved in enough solvent to make 5.00 mL of solution. The osmotic pressure of this solution is measured to be 0.180 atm at 25.0 °C. Calculate the molar mass of the protein. Be sure your answer has the correct number of significant digits. g x10 molarrow_forward
- A solution is prepared at 28°C and its concentration is expressed in three different units: percent by mass, molality, and molarity. If the solution is then heated to 77°C. Which, if any, of these concentration units will change? If none, answer "none".arrow_forwardCalculate the molality of a solution formed by adding 3.40 g NH4Cl to 17.5 g of water.arrow_forwardDifferentiate between 1 molar solution and 1 Molal solutionarrow_forward
- A solution is made using 16.7 percent by mass CH2Cl2 in CHCl3. At 30 °C, the vapor. pressure of pure CH2Cl2 is 490 mm Hg, and the vapor pressure of pure CHCl3 is 260 mm Hg. The normal boiling point of CHCl3 is 61.7 °C. What is the molality of CH2Cl2 in the solution?arrow_forwardWhat is the mole fraction of 25% sucrose solution (C 12 H 22 O 11 )? Find its molality.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY