
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
6.37
Steam undergoes change from an initial state of 450C & 3000kPa to a final state of 140C & 232 kPa.
Determine Delta H & Delta S..... By Equations for an ideal gas...
please help. i pulled up values off steam tables but i dont know if i am on the right direction.....
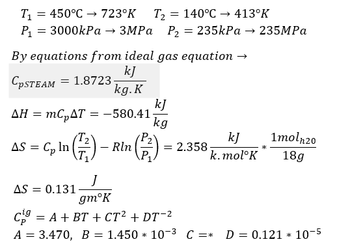
Transcribed Image Text:T₁ = 450°C → 723°K T₂ 140°C →→ 413°K
P₁ = 3000kPa → 3MPa P₂ = 235kPa → 235MPa
By equations from ideal gas equation →
kJ
CpSTEAM 1.8723-
kg. K
AH = mc₂AT = -580.41
AS = Cp In (²/²) -
Rln
AS = 0.131
kJ
kg
kJ
k.mol K
= 2.358-
1moln20
18g
J
gmᵒK
C = A + BT + CT² + DT-²
A = 3.470, B = 1.450 * 10-3 C =* D = 0.121 10-5
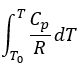
Transcribed Image Text:T
To
Gp ar
-
R
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- 4arrow_forwardQ4. Thermodynamic Cycle (Rankin e cycle and Diesel cycle) (a) Steam having dryness fraction of 55% at 70C temperature. Using steam table, calculate: (i) Specific enthalpy h (kJ/kg) Specific entropy s (kJ/kg-K) (ii) (b) Explain saturated liquid line, saturated vapor line and triple pointarrow_forwardAn adiabatic steam turbine in an electric power plant is designed to accept steam at 5000 kPa and 600°C and discharge the steam at 101.3 kPa. a) What is the exit temperature of the steam and the work produced (per kg of steam) if the turbine operates adiabatically and reversibly? b) If the turbine operated adiabatically but irreversibly, with an isentropie efficieney of 80 %, what would be the exit temperature and the work produced (per kg of steam)? c) For part b, what is the entropy generation Sa?arrow_forward
- Need a b carrow_forward7.49. Operating data are taken on an air turbine. For a particular run, P₁ = 8 bar, T₁ = 600 K, and P₂ = 1.2 bar. However, the recorded outlet temperature is only partially legible; P2 it could be T₂ = 318, 348, or 398 K. Which must it be? For the given conditions, assume air to be an ideal gas with constant Cp = (7/2)R.arrow_forwardShow complete solution and diagram Propylene gas at 127°C and 38 bar is throttled in a steady-state flow process to 1 bar, where it may be assumed to be an ideal gas. Estimate the final temperature of the propylene and its entropy change.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The