
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN: 9780134580999
Author: Elaine N. Marieb, Katja N. Hoehn
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
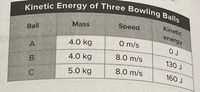
Transcribed Image Text:Kinetic Energy of Three Bowling Balls
Kinetic
Mass
Speed
Ball
energy
4.0 kg
O m/s
A
4.0 kg
8.0 m/s
130 J
B
5.0 kg
8.0 m/s
160 J
C

Transcribed Image Text:regarding kinetic energy is true? sc.7.P.11.2
Based on the table above, which statement
regarding kinetic energy is true? sc.7.P112
A The kinetic energy of an object is
A
O calculated by multiplying mass times
speed.
B The objects at rest will have more
kinetic energy than the objects in
В
motion.
© Objects that have the same mass have
the same kinetic energy no matter how
fast the object moves.
When objects travel at the same speed,
D
the more massive object has more
kinetic energy.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Use the following information to answer questions 20 and 21. Assignment Booklet 4B Two cars, each with a mass of 1000 kg, are travelling in opposíte directionsn is car travelling to the right is travelling 30 m/s, and the car travelling to the lert is travelling 20 m/s. 1000 kg 30 m/s 1000 kg 20 m/s 20. What is the total momentum of the vehicles after they collicde? A. -50 000 kg-m/s B. 50 000 kg.m/s C. -10 000 kg.m/s D. 10 000 kg.m/s al ne 21. If the two vehicles collide and lock together, what is their velocity after the collision? A. -5 m/s Aon s quAua B. 5 m/s elg C. -10 m/s D. 10 m/s Return to page 70 of the Student Module Booklet and begin the Section 3 Review. os elomun ef et birov ort to solniedarrow_forward3. Show the calculation of (1) the expected grams of alum (KAI(SO4)2 12 H2O) formed from the reaction of 1.4 grams of aluminum metal and then show the calculation of (2) the % yield if the actual yield of alum is 21.31 grams. Overall reaction is shown below: 2 Al + 2 KOH + 10 H2O + 4 H2SO4 →2 KAI(SO4)2 12 H2O + 3 H2(g)Tarrow_forwardHow to calculate the theoretical volume of cold airarrow_forward
- Name PS. 3.5 Chemical Reactions 5. Aluminum was first produced in 1825 by Hans Oersted using the following reaction. KCI (s) K (s) AICI, (s) Al (s) 6. Silver is recovered from silver ore by converting the ore into silver sulfate which is then reacted with copper. Cu (s) Ag,S0, (aq) 7. Phosphoric acid is produced at a fertilizer plant. H,SO, (aq) + Ca (PO,)2 (s) 8. Bromine is commercially produced from MgBr, found in sea water. Cl, (g) MgBr, (aq) 9. Hydrogen sulfide (sour) gas from a wild natural gas well reacts with the lead (II) chromate pigment in paint on homes. H,S (g) PbCrO, (s) 10. Hydrogen sulfide gas from a wild sour natural gas well reacts with the silver in cutlery and ornaments in homes. H,S (g) Ag (s)arrow_forwardD Question 3 Observe these two structures and choose the correct statement Но Но A and B have the same boiling point O A is more commonly found in nature compared to B O A and B are isomers O A and B are PUFA « Previousarrow_forwardNonearrow_forward
- The energy released from fission is about 200 MeV per fission event or 3.2-10-11 J per 235U nucleus. The fission of 1 g of 235U generates about 1 MW of thermal power; thus, a reactor that contains 1 kg of 235U fuel generates about 1 GW (109 W). If the reactor core is immersed in a heat bath containing 8.7.105 liters of water (12 m x 6 m x 12 m) initially at 25°C, how long will it take for the water to begin to boil off? Assume that the heat capacity of water is constant and that there is no heat lost from the reactor coolant to the surroundings.arrow_forwardUse the following balanced equation for problems 1–5. Molar masses are given below. 2 As 3 H2 2 AsH3 150. kcal + + molar masses 74.92 g 2.02 g 77.95 g How many moles of hydrogen are required to react with 25.00 moles of arsenic, As ? A. 75.00 mol B. 25.00 mol C. 16.67 mol D. 37.50 molarrow_forwardThe amount of heat in kJ required to convert 0.7 gal of liquid water to steam equal… ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education