
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
physical che
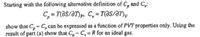
Transcribed Image Text:Starting with the following alternative definition of C, and C,:
C, = T(ƏS/ƏT)p, C, = T(aS/ƏT)y
show that C, – C,can be expressed as a function of PVT properties only. Using the
result of part (a) show that C, – C, = R for an ideal gas.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 6 steps with 14 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 11. State whether the following elements are metals, nonmetals, or metalloids. a. Potassium b. Phosphorus C. Silicon d. lodine 12. Identify the principal type of energy (kinetic or potential) that is exhibited by each of the following. a. A piece of coal b. A falling rock ||| c. A compressed metal spring d. A rolling soccer ball on a level fieldarrow_forward13. Soru Complete the given reaction. Fe(s) + H2SO4(aq) + ........ ........ A) Fe (aq) + SO2(g) B) FeSO4(s) + H2(g) C) FeH2(aq) + H2(g) D) FeH2(aq) + SO2(g)arrow_forwardThe molecular orbital (MO) diagram of OF is given below. Answer Questions 15 – 18. 2p, 2p, 2p, 2p. 2p, 2p. 25 25 OF Farrow_forward
- Q1-Complete the blanks? 1-Major categories of gaseous atmospheric chemical species are................. 2-An atmospheric molecule designated by an asterisk, such as NO2*, is...................arrow_forwardWhat were the challenges that John Dalton went through including how he got their knowledge to be accepted by peers in the scientific communityarrow_forwardtributi X Normal Distributi X ample Work rmat Tools Extensions Help locument/d/1KWoB0hrXolu7UEKbq4f8LBMHfpjHBRVU3qUzVI7K80U/edit Saved to Drive Normal text I 2022-2023 Mr. R X - I ▼ | Calibri 1 16 11. Which quantity is used to define 1 atomic mass unit? 3 100 Copy of Chern B x I UA 4 5 hp 4/27 - Unit 5.mp4 X particles. 12. How many molecules are there in 2 g of water (H₂O)? HINT: Molar mass of water is 18 g/mol Pathway: mass → moles → molecules E Did You Know? The word mole represents a specific number of objects, 6.022 × 10²¹ , just as the word dozen represents a specific number of objects, 12. The molar mass is the mass of a substance that contains 6.022 × 10²¹ DC Normal DE VE E 127 64°F Mosarrow_forward
- 6. A 15.00 g sample of an oxide of nitrogen is heated and decomposes to nitrogen and oxygen. All the oxygen is removed and 3.89 g of nitrogen remains. a. What mass of oxygen was also formed when the sample decomposed?arrow_forwardMass = 1kg, Speed = 2m/s. KE = ?arrow_forwardOrder the theories from oldest to most recent. START ORDER Atoms consist of mostly empty space with a dense nucleus of positive charge. Atoms are tiny indivisible particles that make up all matter. Electrons occupy specific energy levels surrounding a positively charged nucleus. Electrons move about a positively charged nucleus in clouds that are defined by probabilities. Negatively charged electrons are embedded in a mass of positive charge. END ORDERarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY