
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
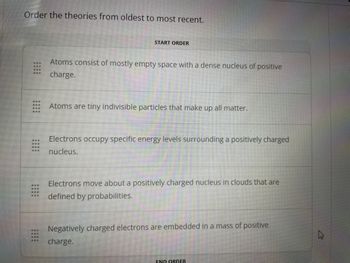
Transcribed Image Text:### Order the Theories from Oldest to Most Recent
1. **Atoms are tiny indivisible particles that make up all matter.**
2. **Negatively charged electrons are embedded in a mass of positive charge.**
3. **Atoms consist of mostly empty space with a dense nucleus of positive charge.**
4. **Electrons occupy specific energy levels surrounding a positively charged nucleus.**
5. **Electrons move about a positively charged nucleus in clouds that are defined by probabilities.**
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I need Helparrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardChoose the appropriate pair of atoms that contain the same number of neutrons. (Example: ^13 6C = 136C, ^13 7N = 137N and so on) Group of answer choices a. ^13 6C and ^13 7N b. ^37 17Cl and ^36 18Ar c. ^18 8O and ^19 9F d. ^6 3Li and ^7 3Liarrow_forward
- MODULE 6 A STUDENT ACTIVITY MAKING AN IMAGINARY PERIODIC CHART INTRODUCTION The following 26 elements were discovered in another far-away galaxy. They are symbolically represented by the letters of our alphabet and are listed in the order of their discovery. The intrepid explorers were able to determine the atomic masses of the elements and to group them partially into families of elements. PURPOSE • To design your own periodic chart. DATA OBTAINED BY THE EXPLORERS The Atomic Masses of the Elements in the Far-Away Galaxy A=45, B=47, C= 59, D=1, E=3, F= 13, G= 25, H= 15, I= 5, J= 7, K= 17, L= 49, M= 39, N= 21, O= 19, P= 9, Q= 11, R= 29, S= 50, T= 62, U= 63, V=53, W= 43, X= 33, Y=23, Z= 35 The Groups/Families of the Elements Active Gases: GTDL Liquids: S M E U H Metallic Solids: I K V I. I. III. IV. Transition Solids: JOR W Nonmetallic Solids: X P Noble Gases: Q ZCY B V. VI. Not yet classified into groups: F AN PROCEDURE 1. Using data obtained by the explorers, try to arrange the…arrow_forwardResearchers from Lund University in Sweden created it by slamming atoms of one element, calcium, into atoms Scientists confirm new element 115 after atoms collide By Ben Brumfield (1) As though it wasn't hard enough to memorize the names and atomic weights of 117 elements in the periodic number of 115 on the periodic table, the list of all elements known to humanity. table, scientists have now confirmed a new one. of another called americium. . Use paragraph 1 and 2 to describe how the element with 115 protons was createc. (3)The Swedes were the second groun of scientists to create the element. A group of Russian scientists put together the same type of atom in 2004. (4)But the new experiment corroborated their work and confirmed 115's existence. Still, this doesn't mean that you'll see element 115 on the next periodic table poster that gets published. The discovery still has to be approved by a committee composed of members of the International Union of Pure and Applied Chemistry and…arrow_forwardal pages that helped with this content 88 Element Symbol H He Li Be B C Atomic Number 2 01 11451 Number of protons 1 2 Total number of electrons 1 2 (ve weh Shell Model n = 1, 2, 3, ... OM LUISUA G COLOUR CHE U SU GUS 1566 llada) er darge 11 si no wo miestod auspun orld mo 329woldat bibohogart no wos obleas idat a GUSLOA ( ET VIL SISCIA SOMAG GUS COCH LOA leborn and to poh topt uger apon Es Core Electrons 0 be owe Suc Jaum duob Jeurn yhodomos pro 29mco bicort nor over by 11,aphild stil 31 to S Valence electrons LAUK ratall 1 2 ncpris-Euro Lebusura por enc Column on the periodic table Lewis dot structure 1H: 8 He: Li Simplified electron arrangement 1e 2e He is special small-so not octet, but full. Will electrons be lost or gained to complete the complete the outer shell? lose le Usual ionic charge 1+ Noble gas-full outer shell-will not lose or gain e- For this class C family will only share e- no ionsarrow_forward
- Explanation on paper to write it down so its eaiser to understandarrow_forwardD. Match items from column (A) with the suitable one from column (B) (A) (В) Alkaline earth metals Са 1 2 Noble gases Со 3 Halogens Ва Alkali Metals 4 Pb 5 One of d-block elements Na One of s-block elements +2 One of p-block elements Kr 7 Alkali metal located in period 3 K* 8 Noble gases located in period 4 Group 1A 10 Charge of ions of group IIA Group 2A Isoelectronic with Ar Group 7A 11 12 Similar chemical properties to F Group 8A Isoelectronic with Ne CI 13 Similar chemical properties to Mg 14arrow_forwardDoes any element with ZS 92 match the description? If you checked yes, give the symbol of an element with Z S 92 description that matches. An element in Period 4 and Group 3A. O Yes O No A metalloid in Group 5A. O Yes O No A halogen with a higher atomic number than silicon. O Yes O No A main-group element in Period 2. O Yes O No Oarrow_forward
- Can you help with 17 and I showed you an answer from the answer book. I think the legend of the colors of the species might be incorrect. Does this make sense. ?This is not a graded question as it is a practice question . I am 60 years old and helping my son prepare for the AP exam in a few months. We do questions at the back of the textbook by Zumdahl and Zumdahlarrow_forwardChrome File Edit View History Bookmarks Profiles Tab Window Help Purple/Black Iridescent - KPM X wilmington postal code - Goo X Dealers - Ni A www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IvdWKW_BBZZIE O ATOMS, IONS AND MOLECULES Counting protons and electrons in atoms and atomic ions Fill in the missing information: symbol atom or ion? number of number of check all that apply protons electrons O neutral atom V cation anion 21 20 neutral atom O cation O anion 14 Te O neutral atom cation V anionarrow_forward25. Classify each of the following elements as a (an) alkali metal, alkaline earth metal, transition metal, halogen, noble gas, or metalloid. Draw lines connecting elements in this table that have similar properties. a. sodium b. germanium c. calcium d. fluorine e. xenon f. copper g. chlorine h. silicon i. potassium j. magnesiumarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY