
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Saponification of Wintergreen Oil
The purpose of this experiment was to convert wintergreen oil into salicylic acid using an aqueous base while also learning how to hydrolyze esters under basic conditions, reflux a reaction, and perform a vacuum filtration.
Practise Question
1. Based on the data determine the yield and melting point.
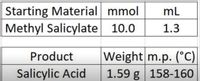
Transcribed Image Text:Starting Material mmol
Methyl Salicylate 10.0
mL
1.3
Weight m.p. (°C)
1.59 g 158-160
Product
Salicylic Acid
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 5. Synthesis of aspirin a. Write the sequence of reactions to produce aspirin using salicylic acid, acetic anhydrous, and phosphoric acid.arrow_forwardQuestion 1 The amount of aspirin synthesized in the lab is proportional to the quantity of salicylic acid used amount of concentrated sulphuric acid used quantity of acetic anhydride used quantity of ethanoic acid used Moving to the next question prevents changes to this answer.arrow_forwardGiven that the nucleophilic substitution reaction used 5.0 mL of t-pentyl alcohol and 12.0 mL of conc. hydrochloric acid to produce t-pentyl chloride, what is the theoretical yield of t-pentyl chloride, in grams? Please report your answer to the correct number of significant figures; do not include units. IUPAC name of t-pentyl chloride is 2-chloro-2-methylbutane.arrow_forward
- An alcohol + a carboxylic acid combine to form an________; the small molecule that is lost in joining the alcohol and carboxylic portions together comes from alcohol -H and carboxylate -OH, to form a molecule of_______.arrow_forwardCreate balanced chemical reactions for the following reactions. Organic reactions should be written with the line-angle drawing of each structure. 1. Reacting salicylic acid with acetic anhydride to form acetylsalicylic acid, with the use of sulfuric acid as a catalyst. 2. Water reacting with acetic anhydride to form acetic acid.arrow_forwardA student found an unknown solution which had the distinct odor of an organic compound. In an attempt to identify the functional group, she performed several of the tests used in Experiment # 6. The observations are tabulated below. a. Primary alcohol b. Carboxylic acid c. Tertiary alcohol d. Secondary alcohol e. Aldehyde f. Ketone Reagent Color of control sample Color after the test Potassium dichromate (K2Cr2O7) Orange Green Potassium permanganate KMnO4) Deep Purple Brown precipitate Brady's Reagent (dinitrophenylhydrazine) Red-orange solution Deep red precipitate Legal's reagent (sodium nitropruside) Bright red solution No change observed; no precipitate eitherarrow_forward
- 4. Assume that an esterification reaction is done using 0.10 moles of a carboxylic acid and 1.0 moles of an alcohol. What is the theoretical yield (in moles) for the reaction? (You can write a generic esterification reaction to help answer this question and recognize the limiting reagent.) 5. A student completes the reaction described in question 4 and obtains 5.3 g of the product (the product has a molecular mass of 74.0 g/mol). a) What is the actual yield (the moles of product obtained)? Show your calculation, including units. 5.3 g product X mol products b) Calculate the % yield for the reaction. Show your calculation, including units.arrow_forwardWhen setting up an organic reaction for the first time, a concentration of reactants in tens to hundreds of micromolar range (mM) is often required. How much solvent (DCM) is required to prepare a solution that has a reactant concentration of 200mM? a) 0.126mL b) 1.26mL c) 12.60mL d) 126mL e) None of the options is correct.arrow_forwardYou have a mixture of an aryl halide and a carboxylic acid that you wish to separate(both are solids). Both are soluble in diethyl ether. Explain ALL the steps you would take to obtain the two compounds in pure form from your sample.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY