
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Indicate which dots are mistakes ty
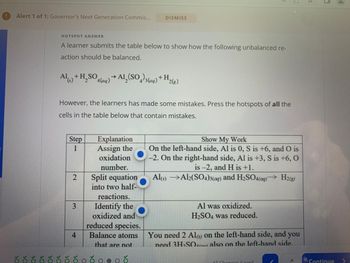
Transcribed Image Text:Alert 1 of 1: Governor's Next Generation Commis...
HOTSPOT ANSWER
A learner submits the table below to show how the following unbalanced re-
action should be balanced.
Al(s) + H₂SO4 (aq) → Al₂(SO4)3(aq) + H 2(g)
However, the learners has made some mistakes. Press the hotspots of all the
cells in the table below that contain mistakes.
Step
1
2
3
Explanation
Assign the
oxidation
number.
4
Split equation
into two half-
reactions.
Identify the
oxidized and
reduced species.
Balance atoms
that are not
á ð ó ó ó ó ó óoðonoð
DISMISS
J
Show My Work
On the left-hand side, Al is 0, S is +6, and O is
-2. On the right-hand side, Al is +3, S is +6, O
is -2, and H is +1.
Al(s)
Al2(SO4)3(aq) and H₂SO4(aq)→ H2(g)
Al was oxidized.
H₂SO4 was reduced.
You need 2 Al(s) on the left-hand side, and you
need 3H₂SO
also on the left-hand side.
K
Continue
De
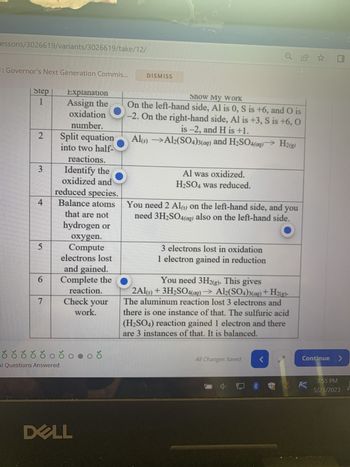
Transcribed Image Text:Alert 1 of 1: Governor's Next Generation Commis...
HOTSPOT ANSWER
A learner submits the table below to show how the following unbalanced re-
action should be balanced.
Al(s) + H₂SO4 (aq) → Al₂(SO4)3(aq) + H 2(g)
However, the learners has made some mistakes. Press the hotspots of all the
cells in the table below that contain mistakes.
Step
1
2
3
Explanation
Assign the
oxidation
number.
4
Split equation
into two half-
reactions.
Identify the
oxidized and
reduced species.
Balance atoms
that are not
á ð ó ó ó ó ó óoðonoð
DISMISS
J
Show My Work
On the left-hand side, Al is 0, S is +6, and O is
-2. On the right-hand side, Al is +3, S is +6, O
is -2, and H is +1.
Al(s)
Al2(SO4)3(aq) and H₂SO4(aq)→ H2(g)
Al was oxidized.
H₂SO4 was reduced.
You need 2 Al(s) on the left-hand side, and you
need 3H₂SO
also on the left-hand side.
K
Continue
De
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Chemistry How to balance centrifuge with odd number of vials with no balancing vials. Given 24 slots, with 7, 9, 11, 13, 15, 17, 19 vials.arrow_forwardIs cm the same as cm3?arrow_forwardExplain why materials with very low density exhibit a greater degree of uncertainty in mass when weighed on the analytical balance?arrow_forward
- Which of the following are important sources of error in cartographic products? Select one: OA. Generalization OB. Digitization OC. Annotation OD. Fuzzy boundariesarrow_forwardNeed help with homeworkarrow_forwardA method of analysis yields masses of gold that are low by 0.4 mg. This method is used for analysis of ores that assay about 3.1% gold. What minimum sample mass should be taken if the relative error resulting from a 0.4-mg loss is not to exceed a. -0.1%? Mass = b. -0.4%? Mass = 6. C. -0.8%? Mass = %3D d. -1.1%? Mass =arrow_forward
- What characteristics of a fired bullet are individualizing? The individualizing characteristics of a fired bullet are : caliber, rifling pattern, direction of twist of the lands and grooves, number of lands and grooves. IS MY ANSWER CORRECT?arrow_forward22.9 mol Hg Express your answer using three significant figures. ΑΣφ N Submit Request Answer Part D 0.280 mol Na Express your answer using three significant figures. ΑΣΦ %3Darrow_forwardSimplify the following units:kgm3/ms2g/cm3smL/cm3s2/s3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY