
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Write out the steps needed to separate hydrocarbon A and
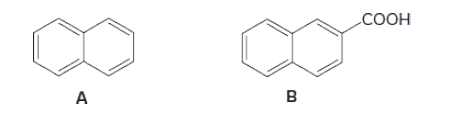
Transcribed Image Text:СООН
A
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the structure of benzyl chloride :arrow_forwardWrite the IUPAC name of the carboxylate salt given below.arrow_forwardFollowing ester (methyl benzoate) was hydrolyzed in presence of an acid catalyst. This reaction produces --- and ---. Question 28 options: benzoic acid, ethanol benzoic acid, water acetic acid, benzene benzoic acid, methanolarrow_forward
- B Which statement BEST describes the distinction between a hydration and a dehydration reaction? a) Hydration requires a carbonyl group, and dehydration does not. b) Hydration is the addition of water, and dehydration is the loss of water. c) Hydration results in breaking bonds, and dehydration creates bonds. d) Hydration is an oxidation, and dehydration is a reduction. e) Hydration and dehydration actually describe the same process.arrow_forwardOrganic Chemistry Маxwell Draw the organic and inorganic products for the following acid/base reaction. H .N. Н. 2 products H.arrow_forward6. Phenobarbital is a long-acting sedative, hypnotic, and anticonvulsant. a) Name all functional groups in this compound. b) Draw structural formulas for the products from complete hydrolysis of all amide groups in aqueous NaOH. H -N N H Phenobarbitalarrow_forward
- Amines with more than 6 carbons are soluble in: a) aqueous HCI b) aqueous NaHCO3 d) water c) aqueous NaOH Which of the following would give a positive iodoform test? acetone a) benzophenone c) 3-pentanone d) cyclopentanone meth "Saponification" as the term is used in organic chemistry means: a) acidic hydrolysis of an ester b) basic hydrolysis of an ester c) acidic hydrolysis of an amide d) basic hydrolysis of an amide 3. ( Propylamine can be synthesized by the LiAlH4 reduction of: a) CH3CH2CECH b) CH3CH=NH d)) CH3CH2CEN c) CH3CH2NO2arrow_forwardComplete the following syntheses – they may be two- or three-step processes. Include any necessary catalysts or reaction conditions. a) Prepare propanone from 1-propanolarrow_forward1. Esters can be formed by the condensation of a carboxylic acid with the hydroxyl group of an alcohol or phenol. a) Use structures to show the condensation of acetic acid (IUPAC: ethanoic acid) with the hydroxyl of cyclohexanol. b) Explain why 'condensation' is an appropriate name for this reaction. c) You'll synthesize the ester in aspirin by condensing the phenol group of salicylic acid not with acetic acid, but with acetic anhydride. Explain why the experiment calls for the use of an anhydride, rather than an acid.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY