
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
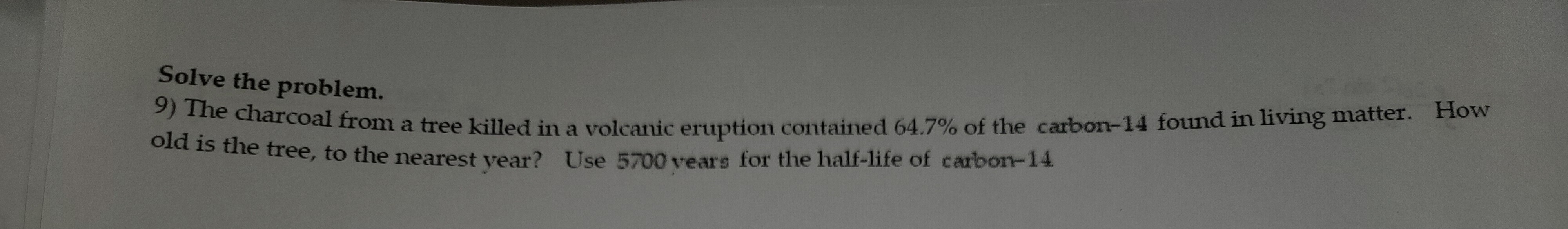
Transcribed Image Text:Solve the problem.
9) The charcoal from a tree killed in a volcanic eruption contained 64.7% of the carbon-14 found in living matter. How
old is the tree, to the nearest year? Use 5700 years for the half-life of carbon-14
Expert Solution
arrow_forward
Step 1
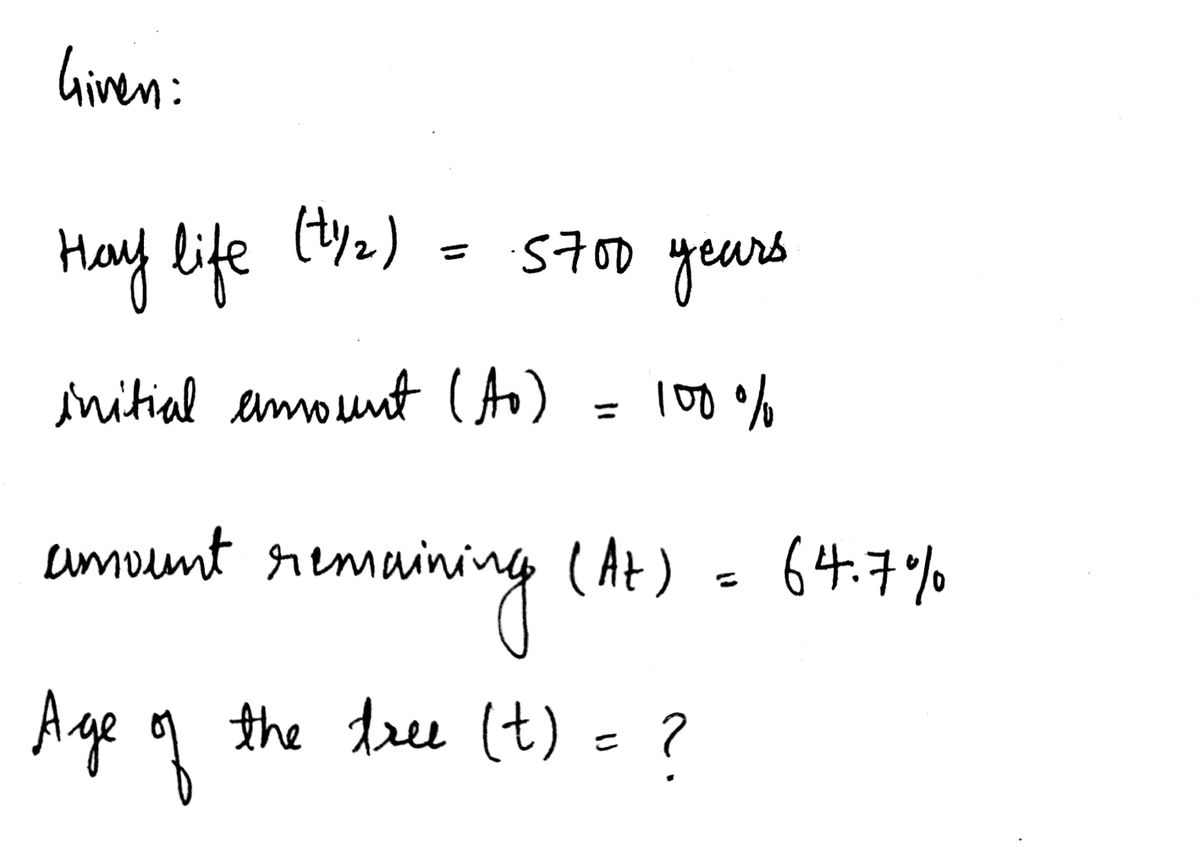
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- need help with this chemistry question, thanksarrow_forwarda) Mrs. Price's son, Liam is currently working as a boilermaker at the Darlington Nuclear Power plant. He is working with Uranium-237 which has a half-life of 13.75 days. What mass of an initial sample of 567.5g of uranium-237 would remain after 56 days? b) Write the complete reaction for the decay of Po-210 if it undergoes 2 consecutive alpha decay followed by a beta decay followed by another alpha decay.arrow_forwardNonearrow_forward
- Carbon-14 has a half-life of 5730 yr. A living organism has an activity of 15.2 counts per minute (cpm) per gram of carbon. If a bone is determined to have an activity of 10.5 cpm per gram of carbon, how old is the bone in the unit of years? Please report an integer.arrow_forwardPlease send me the question in 20 minutes it's very urgent plzarrow_forward3.) The half-life of 14C is 5730 years. A sample contains 90.8 % of the 14C that it would contain if it were still part of a living being. What is the age (in years) of this sample?arrow_forward
- If a sample of radioactive material undergoes 1.0134E13 decay events in 83 seconds, how many Curies are contained in the sample?arrow_forwardA wooden artifact from an ancient tomb contains 30 percent of the carbon -14 that is present in living trees. How long ago, to the nearest year, was the artifact made?( The half-life of carbon-14 is 5730)arrow_forwardCarbon-14 has a half-life of 5730 yr. A living organism has an activity of 15.2 counts per minute (cpm) per gram of carbon. If a bone is determined to have an activity of 11 cpm per gram of carbon, how old is the bone in the unit of years? Please report an integer.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY