
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
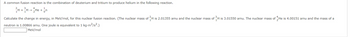
Transcribed Image Text:common fusion reaction is the combination of deuterium and tritium to produce helium in the following reaction.
H + H2He + n
³H
H is 3.01550 amu. The nuclear mass of He is 4.00151 amu and the mass of a
Calculate the change in energy, in MeV/mol, for this nuclear fusion reaction. (The nuclear mass of H is 2.01355 amu and the nuclear mass of
neutron is 1.00866 amu. One joule is equivalent to 1 kg.m²/s².)
MeV/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2 mol of beryllium-8 nuclei combines to form 1 mol of oxygen-16. The mass decrease is 0.0157 g. Calculate the energy released.arrow_forward4. What is the energy released in this nuclear reactionO+n C+He? (The atomic mass of O is 16.999132 u, and that of "C is 14.003241 u) MeVarrow_forwardOne of the most stable nuclei in terms of binding energy per nucleon is 61NI. If the atomic mass of 61Ni is 60.931060 u, calculate the binding energy per nucleon (in J) for 61Ni. (Enter an unrounded value. Assume that the mass of = 1.007825 u, m, = 1.007275 u, m, = 1.008666 u, and m. = 0.000549 u, respectively.) J/nucleonarrow_forward
- Tritium is an isotope of hydrogen that has 1 proton and 2 neutrons with a mass of (3.016amu). If it is formed from fusion between a proton and deuterium (2.014 amu) (1 protonand 1 neutron) how much energy is released?arrow_forwardOne of the many nuclear fission reactions 235U can undergo is:23592U+10n→8934Rb+14458Ce+310nThe total mass of the reactants is 236.0526 amu and the total mass of the products is 235.8765 amu. Determine the energy change when one mole of 235U undergoes this fission reaction in KJ.arrow_forwardFor the fusion reaction shown, calculate the change in energy of the reaction in units of joules per mole. AE= H + H →→→ He + n - J/mol Atom or particle H-1 H-2 H-3 He-3 He-4 neutron Mass (amu) 1.00782 2.01410 3.01605 3.01603 4.00260 1.00866arrow_forward
- a) Mrs. Price's son, Liam is currently working as a boilermaker at the Darlington Nuclear Power plant. He is working with Uranium-237 which has a half-life of 13.75 days. What mass of an initial sample of 567.5g of uranium-237 would remain after 56 days? b) Write the complete reaction for the decay of Po-210 if it undergoes 2 consecutive alpha decay followed by a beta decay followed by another alpha decay.arrow_forwardIn a nuclear reactor, the U-235 atom (235.04393 amu), when hit by a neutron, may split into many possible fission fragments such as Kr-92 (91.92616 amu) and Ba-141 (140.9144 amu), emitting more neutrons. a) Write a balanced nuclear reaction for the fission of U-235 to form Kr-92 and Ba-141. b) Calculate the energy released (in joules per kilogram of U-235) from the above reaction. c) What are two advantages of nuclear power? d) What are two problems with nuclear power?arrow_forwardThe easiest fusion reaction to initiate is 2₁H + ³H → ₂He + ¹on Calculate the energy released, in kJ, per nucleus of 42He produced. The masses of the relevant particles are as follows (1 amu = 1.66×10 27 kg): Particle Mass (amu) H 2.01410 3.01605 4.00260 1.00866 5.4858 x 10-4 H He Inarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY