
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
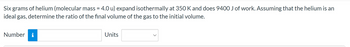
Transcribed Image Text:Six grams of helium (molecular mass = 4.0 u) expand isothermally at 350 K and does 9400 J of work. Assuming that the helium is an
ideal gas, determine the ratio of the final volume of the gas to the initial volume.
Number i
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Any response would be greatarrow_forwardA cylindrical pump contains a fixed number of gas molecules. The piston is free to move, but no gas can enter or leave the pump. The pump is also thermally isolated from the surroundings. Neglect friction between the piston and the cylinder walls. The piston is quickly pressed inward. a) Is the work done on the gas positive, negative or Explain your reasoning. Because it is quick there is little to no energy exchanged meening no work was done. A K zero? b) Is the heat transfer to the gas positive, negative or zero? Explain your reasoning. There is also I heat transfer. c) Did the internal energy of the gas increase, decrease or remain the same? Explain your reasoning. d) Does the temperature of the gas increase, decrease or remain the same? Explain your reasoning. e) Does the pressure of the gas increase, decrease or remain the same? Explain your reasoning. g) What type of process is this? f) Sketch a PV diagram representing this process. (Note: I want to know which axis is P and which…arrow_forward13. The temperatures To of the cold reservoirs and the temperatures T, of the hot reservoirs for four Carnot heat engines are engine 1: Tc = 400K and T₁ = 500K engine 2: T = 500K and T₁ = 600K engine 3: Tc = 400K and T₁ = 600K engine 4: Tc = 600K and TH = 800K Rank these engines according to their efficiencies, least to greatest. A. 1, 2, 3, 4 B. 1 and 2 tie, then 3 and 4 tie C. 2, 1, 3, 4 D. 1, 2, 4, 3 E. 2, 1, 4, 3arrow_forward
- Question A cylinder with a piston contains 0.250 mol of oxygen at 2.40 * 10^5 Pa and 355 K. The oxygen may be treated as an ideal gas. The gas first expands isobarically to twice its original volume. It is then compressed isothermally back to its original volume, and finally it is cooled isochorically to its original pressure. Compute the maximum pressurearrow_forwardDetermine the change in internal energy of a monatomic ideal gas that expands from an initial volume of 1.33 m3 to a final volume of 3.47 m3. During this process the pressure of the gas remains at a constant value of 2.62 105 Pa. Jarrow_forwardThe temperature of 10 moles of an ideal gas is 1000 K. Compute the work done by the gas when it expands isothermally to three times its initial volume. Given: Boltzmann constant: k = 1.38 x 10–23 J/K, Ideal Gas Constant: R = 8.31 J/(mol K) A. 91300 J B. 9130 J C. 913 J D. 91 J E. 9 Jarrow_forward
- An ideal gas expands isothermally, performing 5.00×103 J of work in the process. Part A Calculate the change in internal energy of the gas. Part B Calculate the heat absorbed during this expansion.arrow_forward1 Question 15 According to the first law of thermodynamics, applied to a gas, the increase in the internal energy during any process: 4 O is independent of the heat input O equals the heat input plus the work done by the gas O is independent of the work done on the gas O equals the work done by the gas minus the heat input equals the heat input minus the work done by the gasarrow_forwardOne mole of an ideal gas does 1700 J of work as it expands isothermally to a final pressure of 1.00 × 105 Pa and volume of 0.031 m3 a) what was the inital volume of the gas in cubic meters? b) what is the tempature of gas in kelvinarrow_forward
- The temperature of a monatomic ideal gas remains constant during a process in which 2150 J of heat flows out of the gas. How much work (including the proper + or - sign) is done? number unitsarrow_forwardA gas contained in a cylinder fitted with a frictionless piston expands against a constant external pressure of 1atm from a volume of 5L to a volume of 10L. In doing so, it absorbs 400 J of thermal energy from its surroundings. Determine the change in internal energy of system.arrow_forwardA gas expands from 2.2 L to 3.6 L against a constant external pressure of 1.6 atm. What is the work done? A. -227 J B. 227 J C. 2.24 J D. -2.24 Jarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON