
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
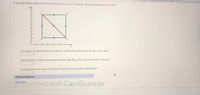
Transcribed Image Text:In the graph below, each unit on the horizontal axis is 5.0 m and each unit on the vertical axis is 5.5 atm.
PA
D.
V
(a) A gas is compressed along the path AC. What is the work done on the gas in this case?
(b) If the gas is instead compressed along the path ABC, what is the work done on the gas?
(c) What is the work done on the gas if the path of compression is along ADC?
Additional Materials
saanned with CamScanner
CRaoing
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A gas is confined in a thermally insulated cylinder as shown in the figure below. The gas is compresses as shown in figure (b). Which one of the following statement is true? a. The work done on the gas will be negative b. The work done on the gas will be Positive c The final pressure on the gas will be zero d. The work done on the gas will be Zero yotarrow_forwardA gas is taken through the cyclical process shown in the figure below. If each unit on the horizontal axis equals 2.75 m³ and each unit on the vertical axis equals 1.25 × 105 Pa determine the net work done by the gas. J J Varrow_forwardA gas follows the PV diagram in the figure below. Find the work done on the gas along the paths AB, BC, CD, DA, and ABCDA.arrow_forward
- The picture shows a pV diagram for an ideal gas in which its pressure tripled from a to b when 804 J of heat was put into the gas. Work done on or by the gas between a and b= 0 W Delta U=804 J a) What is the temperature of the gas at point bb in terms of its temperature at a, Ta?arrow_forwardA gas expands from I to F in the figure below. The energy added to the gas by heat is 302 J when the gas goes from I to Falong the diagonal path. Three paths are plotted on a PV diagram, which has a horizontal axis labeled V(liters), and a vertical axis labeled P (atm). The green path starts at point I (2,4), extends vertically down to point B(2,1), then extends horizontally to point F (4,1). The blue path starts at point I (2,4), and extends down and to the right to end at point F (4,1). The orange path starts at point I(2,4), extends horizontally to the right to point A (4,4), then extends vertically down to end at point F(4,1). (a) What is the change in internal energy of the gas?J(b) How much energy must be added to the gas by heat for the indirect path IAF to give the same change in internal energy?Jarrow_forwardA gas expands from 2.2 L to 3.6 L against a constant external pressure of 1.6 atm. What is the work done? A. -227 J B. 227 J C. 2.24 J D. -2.24 Jarrow_forward
- Please help mearrow_forwardLook at the P-V diagram below (Diagram 1). Calculate the work done by the gas for the paths A, B and C. Assume that in Diagram 1, P1 = 1 atm, P2 = 4 atm, V1 = 5 L, V2 = 15 L. a) WA = 1013 J, WB = 0, WC = -2533 J b) WA = 0.01 J, WB = 0, WC = -0.025 J c) WA = 2533 J, WB = 0, WC = -1013 J Calculate the work done by the gas for the path AB in Diagram 2. Use the data: P1 = 1 atm, P2 = 4 atm, V1 = 5 L, V2 = 20 L. (Path AB is an "isothermal" which means the temperature T is constant on this path). a) 0.012 J b) 1220 J c) 0.0278 J d) 2809 Jarrow_forwardThe graph shown is for a dilute gas that follows the clockwise path of quasi-static steps: isobaric expansion, isochoric reduction of pressure, isobaric compression, and isochoric increase in pressure. The vertical axis is shown in multiples of the pressure pp, where p=2.5atm, and the horizontal axis is shown in multiples of V, where V=4L. Part (a) What is the work done for the segment from state A to state B? Part (b) What is the work done for the segment from state B to state C? Part (c) What is the work done for the segment from state C to state D? Part (d) What is the total work done in making a single clockwise cycle, A to B to C to D?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON