
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
-
Shown in the figure, an insulated rigid tank is divided into two equal parts by a partition. Initially, one part contains an indeal gas, and the other part is evacuated. The partition is then removed, and the gas expands into the entire tank. At the initial state, the mass of the gas is m= 4.00kg, initial pressure is p1 = 600.00 kPa, initial temperature is T1 = 300.00 K. The gas constant is R = 0.7180 kJ/(kg·K). (The internal energy can be determined by the equation ΔU=m·cv·(T2-T1), where cv = 0.7180 kJ/(kg·K) is the specific heat at the constant volume.)
Calculate the internal energy change ΔU.__________ (kJ)
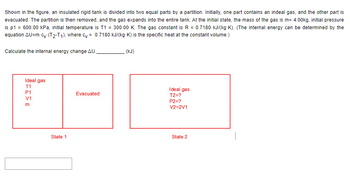
Transcribed Image Text:Shown in the figure, an insulated rigid tank is divided into two equal parts by a partition. Initially, one part contains an indeal gas, and the other part is
evacuated. The partition is then removed, and the gas expands into the entire tank. At the initial state, the mass of the gas is m= 4.00kg, initial pressure
is p1 = 600.00 kPa, initial temperature is T1 = 300.00 K. The gas constant is R = 0.7180 kJ/(kg-K). (The internal energy can be determined by the
equation AU=m-cy (T2-T1), where cy= 0.7180 kJ/(kg-K) is the specific heat at the constant volume.)
Calculate the internal energy change AU.
Ideal gas
T1
FISE
P1
V1
m
State 1
Evacuated
(kJ)
Ideal gas
T2=?
P2=?
V2=2V1
State 2
Expert Solution
arrow_forward
Step 1

Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- A mass of 0.1 kg of helium fills a 0.2 m³ rigid vessel at 350 kPa. The vessel is heated until the pressure is 710 kPa. Calculate the temperature change of helium (in K) as a result of this heating. The gas constant of helium is R= 2.0769 kPa-m3/kg-K. Helium 0.1 kg 0.2 m 350 kPa The temperature change is K.arrow_forwardQ1: A 3-m3 rigid tank contains steam at 225°C. One third of the volume is in the liquid phase and the rest is in the vapor form. Determine (a) the pressure of the steam, (b) the quality of the saturated mixture, and (c) the density of the mixture. Q2:A 1.5 m3 rigid tank that is filled with saturated liquid-vapor mixture at 75 °C is heated slowly. The mass of the mixture is 30 kg. Determine the temperature at which the liquid in the tank is completely vaporized and show the process on the T-v diagram is to be drawn.arrow_forward43.0 g of 40Ar gas are sealed in a container at an initial pressure of 1.50 atm and an initial volume of 0.0500 m3 (state 1). The gas is then made to expand very, very quickly until its volume doubles (state 2). Then it is compressed very, very slowly back to its initial volume (state 3). Show the two processes on a pV diagram, and fill out the table below. p1 = p2 = p3 = V1 = V2 = V3 = T1 = T2 = T3 =arrow_forward
- 43.0 g of 40Ar gas are sealed in a container at an initial pressure of 1.50 atm and an initial volume of 0.0500 m3 (state 1). The gas is then made to expand very, very quickly until its volume doubles (state 2). Then it is compressed very, very slowly back to its initial volume (state 3). Show the two processes on a pV diagram, and fill out the table below. p1 = p2 = p3 = V1 = V2 = V3 = T1 = T2 = T3 =arrow_forwardShown in the figure, an insulated rigid tank is divided into two equal parts by a partition. Initially, one part contains an indeal gas, and the other part is evacuated. The partition is then removed, and the gas expands into the entire tank. At the initial state, the mass of the gas is m= 4.00kg, initial pressure is p1 = 600.00 kPa, initial temperature is T1 = 300.00 K. The gas constant is R = 0.2870 kJ/(kg·K). (The internal energy can be determined by the equation ΔU=m·cv·(T2-T1), where cv = 0.7180 kJ/(kg·K) is the specific heat at the constant volume.) Calculate the final state temperature pressure p2.__________ (kPa)arrow_forwardA piston-cylinder device contains an ideal gas of nitrogen. At the initial state, the volume is V1= 1.00 m3, the pressure is p1= 400.00 kPa, the temperature is T1= 300.00 K. An electric heater within the device is turned on for a time of Δt = 5.00 min. The current is I = 3.00 A, and the source voltage is V = 120.00 V. During the heating process, the gas expands, and a heat loss of Qout = 2.80 kJ occurs. The gas constant is R = 0.297 kPa·m3/(kg·K), and the room temperature specific heat at constant pressure is cp =1.039 kJ/(kg·K). Calculate the mass of the gas, m__________ (kg)arrow_forward
- Krypton in a closed system is compressed adiabatically from 74 K and 1 bar to a final pressure of 24 bar. What is the final temperature in K? Assume krypton is an ideal gas. From Appendix B in the text, we can assume the heat capacity of krypton is independent of temperature and CP=2.5R , where R is the molar gas constant R=8.314 J/(mol K). For an ideal gas, recall CV=CP−R=1.5R. Report your answer in units of K using three decimal places.arrow_forwardQ1arrow_forwardi need urgent i will 10 upvotes .typing onlyarrow_forward
- Six grams of helium(4.0026 g/mol) undergoes an Isothermal process at 127 Celsius, beginning at 6 atm. Their container was found to be 0.04 meters under cube after the expansion. Assuming that helium unit is an ideal gas, calculate the amount of keep that flowed into or out of the helium during the process.arrow_forwardShown in the figure, an insulated rigid tank is divided into two equal parts by a partition. Initially, one part contains an indeal gas, and the other part is evacuated. The partition is then removed, and the gas expands into the entire tank. At the initial state, the mass of the gas is m= 4.00kg, initial pressure is p1 = 600.00 kPa, initial temperature is T1 = 300.00 K. The gas constant is R = 0.2870 kJ/(kg·K). (The internal energy can be determined by the equation ΔU=m·cv·(T2-T1), where cv = 0.7180 kJ/(kg·K) is the specific heat at the constant volume.) Calculate the final state temperature T2.__________ (K)arrow_forwardSolve this question very carefully write clearly and circle the final answer for the” final temperature” in Celsius And The volume of the tank for a final pressure of 10kpa in units of m^3 arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY