
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
2
Transcribed Image Text::相:
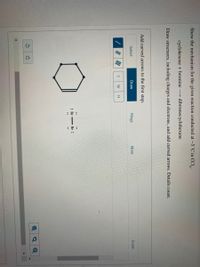
Show the mechanism for the given reaction conducted at -5 °C in CCI,.
cyclohexene + bromine
dibromocyclohexane
Draw structures, including charges and electrons, and add curved arrows. Details count.
Add curved arrows to the first step.
Erase
Select
Draw
Rings
More
C
Br
Br :
2 Q
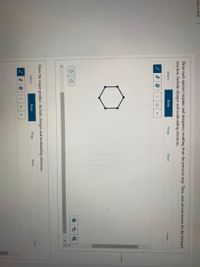
Transcribed Image Text:tion 2 of 15
Draw each species (organic and inorganic) resulting from the previous step. Then, add curved arrows for the forward
reaction. Include charges and nonbonding electrons.
Select
Draw
Rings
More
Erase
C
Br
H
Draw the major product. Include charges and nonbonding electrons.
Erase
Draw
Rings
More
Select
Br
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Milk of magnesia with [H3O+] = 2.0 X 10-9Marrow_forwardA sample of ore contains 0.60% indium by mass. If you want to collect 3.54 dm3 of indium from the ore, what weight (in pounds) of the ore is needed? The density of indium is 7.31 g/cm3arrow_forward1LDA 2) 10. Br OH GMH,SO,/A NH2 11. NH2 12.arrow_forward
- PLEASE SHOW YOUR WORK (THIS IS NOT A GRADED ASSIGNMENT)arrow_forwardWhat is the mass of a piece of silver metal that has a volume of 17.4 cm^3? (The density of silver metal is 10.5 g/cm^3.)arrow_forward3. Derive the expression for the molar mass in terms of the mass, pressure, volume, and temperature of a gas (hint: use the ideal gas law and the definition of molar mass in Equation (2)).arrow_forward
- ZHN 1 eq Ac20 EtzN HOarrow_forwardAn aluminum plate has a diameter of 25.0 cm and weighs 335 g. If the density of aluminum is 2.70 g/cm3, what is the thickness of the plate? (A) 0.632 mm (B) 2.53 mm (C) 3.16 mm (D) 1.58 mmarrow_forwardPart D 36 um Express your answer to two significant figures. ? ΑΣφarrow_forward
- (0.0050 x 28000.0) + (2819 × 15) Express your answer to the appropriate number of significant digits. να ΑΣφ ? (0.0050 x 28000.0) + (2819 × 15) = Submit Previous Answers Request Answer X Incorrect; Try Again; One attempt remaining Part D 861 x [1255 – (3.65 x 101)] Express your answer to the appropriate number of significant digits. ? 861 x [1255 – (3.65 x 101)] = Submit Previous Answers Request Answer X Incorrect; Try Again; 2 attempts remainingarrow_forwardGiven the following molecule on which part of the molecule is the lectern density the greatest? O=C=Oarrow_forward0 2 :Ö:arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY