Question
thumb_up100%
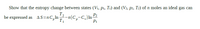
Transcribed Image Text:Show that the entropy change between states (V1, p1, T1) and (V2, p2, T2) of n moles an ideal gas can
T2
be expressed as AS=nC,In-n(C,-C,) In-
T1
P1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Calculate the efficiency of the reversible cycle in figure for an ideal monoatomic gas where the transformation from 1->2 is described by the equation P2 V = A= constant (Assume V2 = 4 V1). P 3 1 V1 2 V2 V Hint: You might need to use the following integral: = √ + GA little bit of a problem can be found for Q1-2 = AU + W where the integral above may result useful to know.arrow_forward1 Point i in Fig. 20-19 represents the initial state of an ideal gas at temperature T. Taking algebraic signs into account, rank the entropy changes that the gas undergoes as it moves, successively and reversibly, from point i to points a, b, c, and d, greatest first. T+AT T-AT Volume Pressurearrow_forwardTwo moles of an ideal gas undergo an irreversible isothermal expansion fromVa=100 liters to Vb=300 liters at T=300K.(a) What is the entropy change for this process?(b) What is the Gibbs free energy change?(c) Is this process spontaneous or not? Explain your answer.arrow_forward
- What is the entropy change for 3.20 mol of an ideal monatomic gas undergoing a reversible increase in temperature from 380 K to 425 K at constant volume?arrow_forwardFind the change in entropy of a monoatomic Van der Waals gas using the equation of state nRT an² an² P = and U = nRT V-nb V2 V (Hint: Start from AU = TAS-pdV)arrow_forwardFigure 20-29 Problem 3.3. *34 Go An ideal gas (1.0 mol) is the working substance in an engine that operates on the cycle shown in Fig. 20-30. Processes BC and DA are reversible and adia- batic. (a) Is the gas monatomic, diatomic, or polyatomic? (b) What is the engine efficiency? Po в /32 E+-+- Vo 2Vo 8 V Volume 16V, Pressurearrow_forward
- Find the work for a system where temperature (T) is constant. (This is called an isothermal process) W = P(V₂ - Vf) W = 0 (V) W = P(V₁ - V₂) W = PV W = - PV) W = -nRT W = nRT W = nRT In W n(V) = nRT Inarrow_forwardAssuming a spherical black body (e=1) of a radius 0.5 m at 300 K is radiating energy into a vacuum near absolute zero, what is the rate of entropy change dS/dt of the black body?arrow_forwardWhat is the entropy change when 0.50 moles of CHCl₃ vaporizes at 61.0 °C? [∆H(vap) = 29.6 kJ/mol at 61.0 °C] ____J/Karrow_forward
arrow_back_ios
arrow_forward_ios