
A cycle starts with an adiabatic compression of the air from state a with a volume V3 to state b with volume V1. At the end of the compression, heat is added (absorbed), resulting in an isobaric expansion to state c with volume V2 followed by an adiabatic expansion back to volume V3 at state d. Finally, heat is expelled, corresponding to an isochoric reduction completing the cycle and bringing air back to state a.
The ratio between the initial and final temperatures for the adiabatic compression is Ta / Tb = 1 / rcγ-1, and the adiabatic expansion is Tc / Td = reγ-1, where the compression ratio rc = V3 /V1 and the expansion ratio re = V3 /V2.
Show that the efficiency of this engine, which only relies on the expansion and compression ratios, is as proved as in figure 1.
.
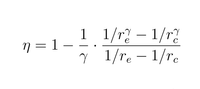
Step by stepSolved in 3 steps with 1 images

- One mole of a perfect gas expands adiabatically and reversibly from 1 L to 5 L in a first process. The same gas is expanded isothermally from 1 L to 5 L in a second process. Which of the following is correct? (i) More work is done during the first process (ii) More work is done during the second process (iii) The amount of work done during both processes is the same (iv) Not enough information to tellarrow_forwardAssume that the internal energy of a system decreases by 300 j while 200 j of work is done by a gas. What is the value of Q ? Is heat lost or gained by the system?arrow_forwardHand written solutions are strictly prohibited.arrow_forward
- Suppose a monatomic ideal gas is changed from state A to state D by one of the processes shown on the PV diagram. PA Isotherms P₂2 atm P₁ atm A kJ E IN T B C 1 1 L I 4.00L 8.00L 16.0L V where P₁ = 1.10 and P₂ = 2.20. mat is the total work done on the gas if it follows the constant-temperature path AC followed by the constant-pressure path CD?arrow_forwardAnswer the question as soon as possiblearrow_forward(a) For the atmosphere, write down an expression for the first law of thermodynamics for an adiabatic process. (b) Write down an expression for change of internal energy of a system in a constant-volume (isosteric) process. (c) Determine the specific internal energy change and the work done during an isosteric cooling from 0°C to -10°C. (d)Determine the pressure level attained when a system expands adiabatically in the atmosphere from 1000 mb and temperature falls from 0°C to -10°C.arrow_forward
- p (kPa) Он 400 5) The figure shows a pV diagram for a cycle of a heat engine for which Q, = 25 J. What is the thermal efficiency of the engine? 200 (Hint: remember to convert cm³ to m³!) A) 17% B) 34% C) 8.5% D) 64% E) 40% 100 V (em³) 200arrow_forwardIn an isochoric process, one mole of an ideal gas of rigid diatomic molecules at volume V is taken from an initial temperature T to a final temperature 4T. Using the convention that heat is positive when it is absorbed by the system, what is the heat transfer in terms of R and T?arrow_forwardA Carnot engine operates between 170°C and 21°C. How much ice can the engine melt from its exhaust after it has done 5.5 ✕ 104 J of work? (The latent heat of fusion for water is 3.33 ✕ 105 J/kg.) kgarrow_forward
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON





