
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
plz solve
(for personal use: 25/30, acidbsconcepts)
Transcribed Image Text:Show Attempt History
Current Attempt in Progress
* Your answer is incorrect.
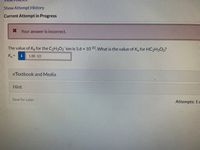
The value of Kp for the C2H3O2 ion is 5.6 x 1010 What is the value of K, for HC2H3O2?
K =
1.8E-10
eTextbook and Media
Hint
Save for Later
Attempts: 1 o
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- er| Portal x Edulastic O Science Spring Semester Exm bapp.edulastic.com/student/assessment/5f18467bc420a100086da 115/cla 28b60984e66etd62fac10/uta/6a9 Elementar. K! Kahoot R ReadWorks Play Quizizz! Newsela Assignm Commontty As ty Play no. Question 5/10 > NEXT BOOKMARK Water is boiled and steam is produced. This is a physical change because the water particles A. have turned into something new. B. are still water particles. C. have reacted with each other. D. have been destroyed. B.arrow_forwardme x C Thermo X Cosmet x C Laptops x M Inbox (7 X C Buy TCL X learn.canterbury.ac.uk/ultra/courses/_19016_1/outline/edit/document/_3537106_1?courseld=_19016_1&view=content T K Saturat X ZA a Amazon x C Show Ye X G what's a X Email- 1 X ☆ b The dia x h EX E ct the rato at which + 4. Consider the interconversion of A and B. Suppose in the absence of an enzyme, the forward rate constant KF is 104 s¹ and the reverse rate constant KR is 106 S¹. Calculate the equilibrium constant K. How would the presence of an enzyme affect the value of K? Karrow_forwardA Gradebook NWP Assessn x NWP Assessn X NWP Assessr NWP Assessn x NWP Assessr NWP Assessn W (1) Messenge x + A education.wiley.com/was/ui/v2/assessment-player/index.html?launchld=6a7d7e2b-ea8d-49fd-af75-e80d6db91765#/question/1 FA 3.9 A-Properties of Gases Question 2 of 7 -/3 View Policies Current Attempt in Progress The motion picture Titanic described the tragedy of the collision of the ocean liner of the same name with an iceberg in the North Atlantic. The ship sank soon after the collision on April 14, 1912, and now rests on the sea floor at a depth of 12607 ft. Recently, the wreck was explored by the research vessel Nautile, which has successfully recovered a variety of items from the debris field surrounding the sunken ship. Calculate the pressure in atmospheres and pounds per square inch exerted on the hull of the Nautile as it explores the sea bed surrounding the Titanic. Pressure = atm. eTextbook and Media Save for Later Attempts: 0 of 3 used Submit Answer 5:20 PM O Type here…arrow_forward
- n Home Work-2 (page 7 of 8) b My Questions | bartleby A moodle.nct.edu.om/mod/quiz/attempt.php?attempt=709737&cmid=76257&page=6 E Apps * Bookmarks تحويل كيلومتر إلى م. .. © E Reading list NCT e-Learning Portal Courses Reports - e-Services Academic Departments - ETC - CIMS - Muayid Mahmood Mohammed Al Azri Fundamentals Of Chemistry (Engineering) Dashboard / My courses / CHEM1100 / Home works / Home Work-2 Quiz navigation Question 7 How many Faraday is needed to deposit 1.8 g of Sodium (Na) from NaCl solution using electrolysis process. Not yet 1 2 3 4 5 6 8 answered Answer: Marked out of Finish attempt . 1.00 P Flag question Time left 191:45:56 Previous page Next page - Submission Link for Home Work-1 Jump to. Quiz 1 for Section-3 - You are logged in as Muayid Mahmood Mohammed Al Azri (Log out) CHEM1100 Data retention summary. Get the mobile app 10:13 PM P Type here to search 72°F Clear O E 1 G 4) ENG 12/14/2021arrow_forwardnalysis Practice Worksheet.docx al Analysis Practice Worksheet.docx (13.5 KB) Page 1 > of 2 4. How much does a cubic meter of water weigh, in pounds? The density of water is 1.00 g/mL. SEP 10 étv MacBook Pro & 6. 7 %3D { R Y 60 * COarrow_forwardHelp 100% 47 T. "ublic Health Ch HSC 258 - Major Projec x MindTap - Cengage Lea X C The Illustration To T =55750828934189288909969212&elSBN=9781305657571&id=D1061392007&nbld=21... * Q Search t Referonces Use the References to access important values if needed for this question. For the following reaction, 50.4 grams of sulfur dioxide are allowed to react with 17.9 grams of water. sulfur dioxide (g) + water (I) sulfurous acid (H2SO3) (g) grams What is the maximum amount of sulfurous acid (H,SO3) that can be formed? What is the FORMULA for the limiting reagent? grams What amount of the excess reagent remains after the reaction is complete? Submit Answerarrow_forward
- °F rtly cloudy m/alekscgi/x/Isl.exe/1o_u-lgNslkr7j8P3jH-lvWyv8WYLP6W0cqJcWJdIACROQwyw24GWHInMM72ts 1 NTBcSzwZOGGhrmIP4yCejeRhX9HIzqlzRTj2iaDBXTCRIHYWNO_-o?10... O CHEMICAL REACTIONS Calculating molarity using solute moles A chemist prepares a solution of mercury(II) iodide (HgI₂) by measuring out 0.0161 μmol of mercury(II) iodide into a 300. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in μmol/L of the chemist's mercury(II) iodide solution. Round your answer to 3 significant digits. µ mol L Explanation Check x10 X Ś Q Search 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use **************** D 1/5 www Jessi Privacy Center | Accessibilityarrow_forwardThird Ma X 占 General A General ( X di General ( N 7C Social x C Clever | P x C Clever | P X E Edulastic x O Mail - AS x A app.edulastic.com/student/assessment/60525b29af74cf0008c2b68f/class/5f20cf3d5dd16ded17965e85/uta/6054999e58acf20009db46a4/it Question 1/19 > NEXT A BOOKMARK The structure of a chromosome is made up of influences our traits. 1 which contains that code for a which A Protein, DNA, Gene B Gene, DNA, Protein C DNA, Genes, Protein O Type here to search DELL 立arrow_forwardWhy is this correct?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY