
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Sharpless
Transcribed Image Text:Sharpless
epoxidation
NaOH, CH;SH
OH
(+)-DET
H,0, (CH2);COH
.SCH5
х
он
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chiral amine A having the R configuration undergoes Hofmann elimination to form an alkene B as the major product. B is oxidatively cleaved with ozone, followed by CH3SCH3, to form CH2 = O and CH3CH2CH2CHO. What are the structures of A and B?arrow_forwardDraw a structural formula for the major organic product of the following reaction: CI- 유 CH2Cl2 CH=CHCOH + Cl2 ⚫ Show product stereochemistry IF the reactant alkene has both carbons of the double bond within a ring. • Do not show stereochemistry in other cases. • If enantiomers are formed, just draw one.arrow_forwardConsider the compound C5H9Br (Compound X). It reacts with potassium tert-butoxide to give Compound Y, C5H8. Compound Y upon reaction with hydrogen produced methylcyclobutane. Compound Y upon reaction with ozone produces one compound with two aldehyde functional groups. Provide the structures of Compound X and Y.arrow_forward
- A compound with formula C8H12 reacts with 2 molar equiv of H₂ to yield a cycloalkane. By treatment of this compound with acidic KMnO4, a dicarboxylic acid, HOOCCH2CH2COOH is obtained. What is the structure of this compound? (A) (B) (C) (D)arrow_forwardPGF 2a is a prostaglandin, a group of compounds that are responsible for inflammation (Section 19.6). (a) How many tetrahedral stereogenic centers does PGF 2a contain? (b) How many double bonds can exist as cis and trans isomers? (c) Considering both double bonds and tetrahedral stereogenic centers, what is the maximum number of stereoisomers that can exist for PGF20? OH HO -CH₂CH=CH(CH₂)3COOH CH=CHCH(OH)(CH₂)4CH3 PGF₂αarrow_forward3 A compound A with a molecular formula CH3O is oxidised with acidified potassium dichromate(VI) to form a liquid B. B reacts with hydrogen cyanide to form a compound C that contains four carbon atoms. Draw the structures of compounds A, B and C. 4 An alkene D with a molecular formula C3H, can be converted into an alcohol E. This alcohol is oxidised to form a compound F, which does not give a silver mirror with Tollens' reagent. Draw the structures of compounds D, E and F.arrow_forward
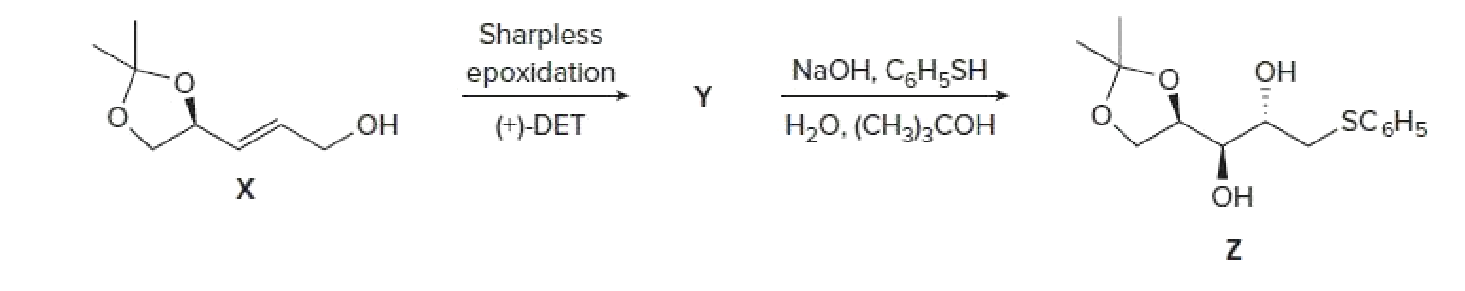
- Sharpless epoxidation of allylic alcohol X forms compound Y. Treatment of Y with NaOH and C6H5SH in an alcohol–water mixture forms Z. Identify the structure of Y and draw a mechanism for the conversion of Y to Z. Account for the stereochemistry of the stereogenic centers in Z. Z has been used as an intermediate in the synthesis of chiral carbohydrates.arrow_forwardCompound A, C₁1 H160, was found to be an optically active alcohol. Despite its apparent unsaturation, no hydrogen was absorbed on catalytic reduction over a Pd/C catalyst. On treatment of A with dilute H₂SO4, dehydration occurred and an optically inactive alkene B, C₁₁H₁4 was produced as the major product. Alkene B, on ozonolysis, gave two products. Product C, C7H6O, was shown to be an aldehyde while product D, C4H8O, was shown to be a ketone. Draw the structure of compound C. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one.arrow_forwardIn addition to more highly fluorinated products, fluorination of 2-methylbutane yields a mixture of compounds with the formula C5H10F2. Draw the structures of all the isomers with the formula C5H10F2 that would be produced and label with a star all the chiral centers present in their structures.arrow_forward
- Compound A, CH10, was found to be an optically active alcohol. Despite its apparent unsaturation, no hydrogen was absorbed on catalytic reduction over a Pd/C catalyst. On treatment of A with dilute H,SO, dehydration occurred and an optically inactive alkene B, CH4 was produced as the major product. Alkene B, on ozonolysis, gave two products. Product C, C,H,0, was shown to be an aldehyde while product D, CHg0, was shown to be a ketone. Draw the structure of compound C. • You do not have to consider stereochemistry. You do not have to explicitly draw H atoms. In cases where there is more than one answer, jtust draw one.arrow_forward2. Show how 1-butanol can be converted into the following compounds: (a) (b) CNarrow_forwardA chemist synthesized compound X as a racemic mixture. When the ketone group in X was enzymatically reduced to the corresponding alcohol, a 100% yield was obtained of the product shown below. Choose the statement that best describes this result. ОН enzyme C;H1 `OCH,CH; pH 4.0 C3H1 `OCH,CH3 ОН ÕH X (racemic) (100% yield) One enantiomer of compound X reacts quickly with the enzyme. The other enantiomer of compound X is unreactive, but rapidly equilibrates with the reactive enantiomer under the reaction conditions. Since compound X was racemic, it makes sense that only a single product was obtained. O The product is a meso compound, so either enantiomer of compound X gives the same product. One enantiomer of compound X reacts quickly with the enzyme, while the other enantiomer of compound X remains unchanged.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY