
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Could you help me with this question? I don't know where to start. All the information has been provided.
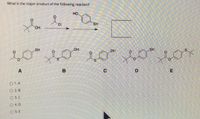
Transcribed Image Text:What is the major product of the following reaction?
HO.
S.
HO.
SH
HO
SH
S.
DI
O 1.A
O 2.B
O 3. C
O4. D
O 5.E
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In 2014 (the last year for which I can find data), Utah County had a carbon monoxide (CO) average concentration of 1.90 ppm. (1 ppm stands for 1 part per million which means in one million volumes of air—choose whatever volume unit you like—there would be one volume of carbon monoxide.) An average human takes inhales 0.50 L per breath and takes 20 breaths per minute. Carbon monoxide in the atmosphere has a density of 1.2 grams per liter. How many liters of air does a person breathe in Utah County in 8 hours? How many liters of carbon monoxide will a person breathe in Utah County in 8 hours? How many milligrams of carbon monoxide would a person in Utah County inhale in an 8 hours?arrow_forwardFill in the Blanks Chemical Price/kg (in dollars) NaOH 21.17 CaCl2 41.28 КОН 31.38 CUSO4 93.32 NH4NO3 59.64 NH4CI 25.04 NaCl 21.15 NaNO3 72.35 Type your answers in all of the blanks and submit Another requirement is that the cost of the salt needs to be evaluated to determine if it is economical to use. To do this, you should consider the price per degree of dropping the temperature of the solution. The table of prices per kg for each salt is shown above. Let's determine the cost for the NaOH solid as an example. We want to determine how much it costs to change the temperature by 1 deg C so we start with the cost given in the table and end with $/deg C. For NaOH: 21.17 $/ 1 kg) X ( 1 kg/ 1000 X ( Type your answer here g/ Type your answer here deg C) Please type your answer to submit Please type your answer to submit Type your answer here $/deg Carrow_forwardThe U.S. Government has about 1.23 * 107 pounds of gold in Fort Knox. How much is it worth at today's value of $1,576.30 per Troy ounce? Given: 12 Toz = 1 Lb Group of answer choices I honestly do not know; however, if it were not for James Bond, the Goldfinger would have stolen all of it. $1.94 * 10^10 US dollars $2.33 * 10^11 US dollars $ 4.30 * 10^-12 US dollarsarrow_forward
- You have a mole of ping pong balls and want to ship them to a near-by town. You have packing boxes that measure 24 inches by 24 inches by 18 inches. How many boxes do you need to pack up all the ping pong balls? The radius of a ping pong ball is 20 mm. If you make any approximations or assumptions, please list them.arrow_forwardA mixture has three components. The percentage of sand was determined to be 37.31 %, the percentage of benzoic acid was determined to be 37.45 %, and the percentage of salt was determined to be 31.69 %. Calculate the total percent recovery. Include the % unit and two decimal places in your answer.arrow_forwardIn my on-campus Organic Chemistry II Lab, my students make aspirin. The melting point in the literature for aspirin is 134-136°C. Suppose the sample of aspirin one of my students made has a melting point range of 129-133 °C. Using this information, answer the following: 1. Do you think the student made aspirin? Why or why not? 2. How could you test your hypothesis? Explain what you would do and what the results would look like if they made aspirin versus if they didn't.arrow_forward
- In 2014 (the last year for which I can find data), Utah County had a carbon monoxide (CO) average concentration of 1.90 ppm. (1 ppm stands for 1 part per million which means in one million volumes of air—choose whatever volume unit you like—there would be one volume of carbon monoxide.) An average human takes inhales 0.50 L per breath and takes 20 breaths per minute. Carbon monoxide in the atmosphere has a density of 1.2 grams per liter. 2. How many liters of carbon monoxide will a person breathe in Utah County in 8 hours?arrow_forwardIn 2014 (the last year for which I can find data), Utah County had a carbon monoxide (CO) average concentration of 1.90 ppm. (1 ppm stands for 1 part per million which means in one million volumes of air—choose whatever volume unit you like—there would be one volume of carbon monoxide.) An average human takes inhales 0.50 L per breath and takes 20 breaths per minute. Carbon monoxide in the atmosphere has a density of 1.2 grams per liter. 3. How many milligrams of carbon monoxide would a person in Utah County inhale in an 8 hours?arrow_forward4 The mixture used in today's experiment was prepared as a 1:1:1 mixture (by mass) of sand, salt, and salicylic acid. Do your results for the mass of each recovered solid indicate a 1:1:1 composition of the mixture? Explain your response.arrow_forward
- What are the sources of these raw materials: 500 g calcium polysulphide and 1.5 kg hydrochloric acid? Additionally, determine whether each raw material is natural or synthetic.arrow_forward3-4 sentences 1. Briefly state what separates science from opinion? 2. Water is the most important molecule to life on this planet. Briefly explain why water has the unique properties it does (high boiling point, high freezing point, high capacity of heat, etc.), especially for a small molecule. 3. How does the depth of water affect light availability? How deep does light penetrate ocean water? How might this affect ocean life? 4. The world oceans are dynamic. One reason is because the oceans are in constant motion. One means of water motion are waves. What are waves? What are three factors that affect wave height?arrow_forward2. You purchase a rectangular piece of metal that has dimensions 5.0 X 15.0 X 30.0 mm and mass 43.43 g. The seller tells you that the metal is gold. To check this, you compute the average density of the piece. What value do you get? Were you cheated?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY