
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
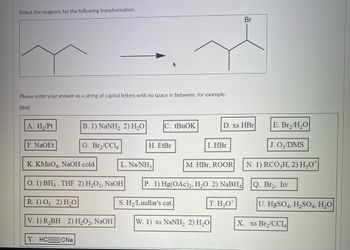
Transcribed Image Text:Select the reagents for the following transformation.
Please enter your answer as a string of capital letters with no space in between, for example:
BME
A. H₂/Pt
F. NaOEt
B. 1) NaNH, 2) H,O C. tBuOK
G. Br₂/CC14
K. KMnO4, NaOH cold
O. 1) BH3. THF 2) H₂O2, NaOH
R. 1) 03 2) H₂O
V. 1) R₂BH 2) H₂O2, NaOH
Y. HC CNa
H. EtBr
L. Na/NH3
S. H₂/Lindlar's cat.
I. HBr
D. xs HBr
M. HBr, ROOR
P. 1) Hg(OAc)2, H₂O 2) NaBH4
T. H30
W. 1) xs NaNH, 2) H,O
Br
E. Br₂/H₂O
J. 03/DMS
N. 1) RCO3H, 2) H3O*
Q. Br₂, hv
U. HgSO4, H₂SO4, H₂O
X. xs Br₂/CC14|
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- CH3 CH, CH, hv CH HyC CHs or CH C H2 X-F, CI, Br or I H,C CH H,C Summarize the effect of each factor on the ratio of the two products shown above. The type of H (benzylic, allylic, 3°, 2°, 1° or methyl). a. b. The number of H's of a given type. C. The identity of X (F, CI, Br, or I).arrow_forwardPropose an efficient synthesis.arrow_forwardQuestion 14arrow_forward
- HW6 #12arrow_forwardIdentify the correct reagents and conditions to perform this chemoselective reaction: H ရှုံး H Select one: Me e. Reagent(s) and conditions? a. NaBH4 b. BH3 THF Pd/BaSO4, H₂ (1 atm.), quinoline, Pb(OAC)2, MeOH d. Pd/C, H2 (1 atm.), MeOH DIBAL Me H H OHarrow_forwardPlease double-check my answerarrow_forward
- Answer b,d,e part onlyarrow_forwardSynthesis also label any major/minor/trace productsarrow_forwardConsider the following reaction. Using the formula of the base and the E2 conditions listed in the reaction below which of the following products will be formed? CH3 CH3 CH2 H3C H3C H3C NaOMe H3C- H3C- H3C- E2 conditions TE F11 F8 F5 F6- Esc F4 & # 4 6 7 1 3 Y E ab H J K D =LK C V B N M Alt Fn טא 当arrow_forward
- What is the reagent for this transformation?arrow_forwardMay you please help me with this ochem question?arrow_forwardEnter your answer as a string of letters in the order that you wish to use the reagents, i.e. bde. Do not use punctuation. Reagents available f. H₂, Pd/C g. (sia)2 BH then H₂O₂, NaOH h. BH3 then H₂O2, NaOH d. Na, i. excess NaNH₂, then H₂O NH3 (1) e. H₂, Lindlar's catalyst j. H₂SO4, H₂O, HgSO4 a. Br₂, CCl4 b. excess NaNH, C. CH₂I Conversion #1: Conversion #2:arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY