
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
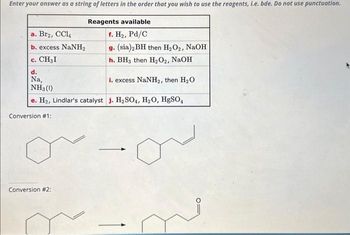
Transcribed Image Text:Enter your answer as a string of letters in the order that you wish to use the reagents, i.e. bde. Do not use punctuation.
Reagents available
f. H₂, Pd/C
g. (sia)2 BH then H₂O₂, NaOH
h. BH3 then H₂O2, NaOH
d.
Na,
i. excess NaNH₂, then H₂O
NH3 (1)
e. H₂, Lindlar's catalyst j. H₂SO4, H₂O, HgSO4
a. Br₂, CCl4
b. excess NaNH,
C. CH₂I
Conversion #1:
Conversion #2:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- a. 8. b. OH Fill in the starting material for the following reaction. ? 1. NaOH 2. HgO+ 人無 3. Hy / Raney Ni C. . Br d. 己 e. 己 OHarrow_forward0-H Oxone/ NaBr. 13.arrow_forward3.) Which arrangement of bonded atoms could explain the high solubility of Compound X in water? IL IV. CH H H CH, H. A. II & III B. III & IV С. I & V D. I onlyarrow_forward
- 40 mg of sodium hydroxide is required to saponify 1gm sample, this means that (MW of NaOH= 40 g/mole, MW of KOH is 56g/mol) O a. saponification value equals 56 O b. acid value equals 40 O C. Acid value equals 56 O d. saponification value equals 40arrow_forwardPlease don't provide handwriting solutionarrow_forwardProvide the appropriate product for the examplearrow_forward
- Select one for each boxarrow_forwardI'm needing help with problem 4, please. Thank you!arrow_forward6. Complete the following reactions by filling what is left out; either the reactants, starting material, or the major product. a. b. C. CI 1. Excess NaNH, 2. H₂O 3.HgSO4 4. NaBH₁, NaOH 1.NAOMe 2.03 3. Zn, H* Coquearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY