
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Kk.106.
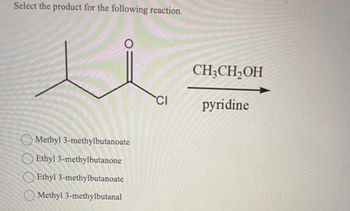
Transcribed Image Text:Select the product for the following reaction.
u
Methyl 3-methylbutanoate
Ethyl 3-methylbutanone
Ethyl 3-methylbutanoate
Methyl 3-methylbutanal
CI
CH3CH₂OH
pyridine
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Anhydrous CuSO4 is converted to CuSO4・xH2O to absorb water from certain liquids. What is the value of x if 24.3 g of anhydrous CuSO4 is required to remove 13.7 g of H2O from a gallon of gasoline? Relative atomic masses:H = 1.008; O = 15.999; S = 32.06; Cu = 63.546arrow_forwardework Due in an Octasulfur(Sg) is the most common allotrope of sulfur and is widely used in the chemical industry, especially in the production of petroleum. While Sg is the main component of elemental sulfur on earth is can also be produced in the laboratory from the reaction of sulfur dioxide with hydrogen sulfide. The balanced chemical equation for this reaction is shown below: 8SO (g) + 16H25(g) + 3S8(s) + 16H20(g) Consider the reaction of 93.0 grams of SO, with 93.0 grams of H,Sto answer the questions below. Drag and drop options on the right-hand side and submit. For keyboard navigation. SHOW MORE v What is the limiting reagent? H,S What is the excess reagent? 87.1 grams How many grams of Sg can be formed? = 131 grams How many grams of excess reagent are left over? 5.77 grams [3 Fulls 10:38 PM-arrow_forward(d) the ion with 74 electrons, 116 neutrons, and a +3 chargearrow_forward
- Three samples (A, B, and C) of pure substance composed ofsodium, sulfur, and oxygen were isolated and purified. Elemental analyses showed that sample-A contained 1.62 g sodium, 1.13 g sulfur and 2.25 g oxygen; sample-B contains 1.60 g sodium,2.23 g sulfur, and 1.67 g oxygen; while sample-C contains 1.46 g sodium, 1.02 g sulfur, and 2.03 g oxygen. Determine whether A, B,and Care samples of the same or different compounds.arrow_forwardWhich of the following pairs react to form ioniccom-pounds: (a) Cl and Br; (b) Na and Br; (c) P and Se; (d) H and Ba?.arrow_forward7. Balance the following equations: a) For the synthesis of urea, a common fertilizer CO2(g) + NH3(g) → NH₂CONH2(s) + H2₂0 (1)arrow_forward
- Br2, Fearrow_forwardWhat is the major product of the following reaction? Br NaOCH 3 heat yarrow_forward4. The “alum” used in cooking is potassium aluminum sulfate hydrate, KAl(SO4)2 ∙ x H2O. To find the value of x, you can heat a sample of the compound to drive off all of the water and leave only KAl(SO4)2.Assume you heat 4.74 g of the hydrated compound and that the sample loses 2.16 g of water. What is the value of x?arrow_forward
- 4 The reaction between NH3 and O2 produces NO and H₂O. 4 NH3 (8) + 5 O₂ (g) 24 NO(g) + 6 H₂O (1) If a complete reaction of a mixture of NH3 and O₂ that had a total mass of 250 g produced 60 g of liquid water, what will be the most likely mass of all the gases remaining in the container after the reaction? Assume that reaction happens in a closed container. 190 g 60 g More information is needed to decide 310 garrow_forwardMN IN C. B. H. K. %3D R. P. 96. 5. 4. 08. 7. 23 %24 > MacBook Pro O CẠH12N O C4H12N2 NÓH®ƆO O CGH16N2 molecular formula? A 115.95 g of a sample is found to contain 71.95 g C, 16.13 g H, and 27.87 g N. If the mw is 116.21 g/mol, then what is its 20 of 20arrow_forwardWhat does xH,0'represent in the formula AL(S0,)²xH,O? The student heats 12.606g of AL(SOxH,0 crystals to constant mass. The anhydrous aluminium sulfate formed has a mass of 6.846 g. Use the student's results to calculate the value of x. The molar mass of AL(SO = 342.3gmol-1. %3Darrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY