
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:+
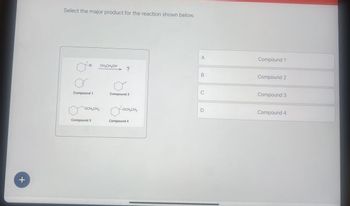
Select the major product for the reaction shown below.
A
Compound 1
Br
CH₂CH₂OH
?
B
Compound 2
C
Compound 1
Compound 2
Compound 3
OCH₂CH₂
-OCH2CH3
D
Compound 4
Compound 3
Compound 4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 6 images

Knowledge Booster
Similar questions
- What is the outcome of the series of reactions shown in the box? OH OCH3 HNO3 Sn or Fe NaNO2 H3CO H2SO4 HCI H2SO4, H2O OCH3 H3CO NO2 A) B) OH HO LOCH3 C) LOCH3 D) H3CO H3CO y-N OH A В ODarrow_forwardWhich of the four options below is a correct product to the following reaction H₂NOC H₂NOC H₂C HN 20 Ho HN CO₂H H₂NOC zo HN NaOH/H₂O CFCOH H₂O+arrow_forwardComplete the reaction map by matching A-E with the given choices. H2 H2 B E Pt Cla Na in lig NH3 он + но Match each item to a choice Aarrow_forward
- Consider the pair of reactions and product options provided below as you respond to the following questions. For each question, select the most correct answer from the provided pull down menu. H3C CH3 H3C CH3 H3C A Br CH3 REACTION A REACTION B H3C CH3 H3C B CI CH3 H3C H₂C What is the most likely product for Reaction A? A H3C CH3 Which reaction proceeds at a faster rate? [Select] CH3 H3C What is the most likely product for Reaction B? [Select] H3C CI O. CH3 CH3 C CH3 CH3 CH3 HCI, 0°C 4 HBr, 0°C H3C Br H3C D CH3 CH3 H3C CH3 насх Based on the reagents and the product you have selected what is the most likely mechanism for both of the reactions? [Select] ♦ E OH CH3arrow_forwardWhat is the major product of the following reactions? Br Br 1) BH3, THF 2) H₂O2, NaOH Br₂, hv H₂SO4, H₂O 1) 0₂ 2) H₂O 1) NaNH/NH3 2) H₂Oarrow_forwardprovide reagents for each steparrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY