
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
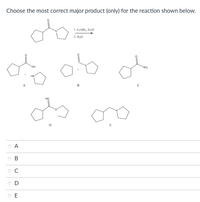
Transcribed Image Text:Choose the most correct major product (only) for the reaction shown below.
1. LIAIH, EtO
2. H,0
NH
HN
B
HN
D
E
A
B
O D
O E
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The reaction in the box is missing a reagent/reactant... NCH3 NHCH3 Which of the following would you use to convert the starting material into the product in high yield? A) An oxidation reagent such as H2CrO4 B) A base such as NaOH/H20 C) An acid such as H2SO4 D) A reduction agent such as NABH4 A В C O Darrow_forwardxviiarrow_forwardWhich of the following is the major product of the reaction shown below? * H 1. CH,MgBr, diethyl ether 2. H,O* CH,CH,CH3 CH3 H f-CH3 -CH,CH,CH3 OH --CH,CH,CH3 HO OH CH3 --H- -H --CH,CH,CH3 -CH3 CH,CH,CH3 HOarrow_forward
- 2. Give the Major and minor products for the following two reactions. Br NaOH H20 D Br Bu-OK 'Bu-OH, Aarrow_forwardHBr ? What is the structure of the major product of the reaction? °୪ Br .8 Br ○ Br -Brarrow_forward3. Which of these two reactions would go better? Why? N2OH CH,CH;Br CH,CH,OH Br CH,CH, CH, NaOH он CH,CCH, CH,arrow_forward
- Which reaction would produce the proposed product in a good yield? H NaBH, MeOH Н,О, Н,О OH НО ОН Н НО, Н,О 1. CH,MgBr 2. 1,0 HO OH X НО ОН хarrow_forwardWhat are the major products of these reactionsarrow_forwardWhat is the name of the product in the following reaction? TRUE or conc. KMnO4, heat FALSE. The reactions shown below will produce an aldehyde. CO,H 1. SOC1₂ 2. LIAI[OC(CH3)3]3Harrow_forward
- For the reaction sequence shown, what is the expected major product? 1. HBr 2. NaOMe ? X X X || ||| IV Jarrow_forwardPredict each product for the following multistep reaction. If ortho/para products are both formed, use the para product in the next reaction. 46. Predict each product for the following multistep reaction. If ortho/para products are both formed, use the para product in the next reaction. Br2 FeBr3 HNO3 A H2SO4 B Fe C HCI NaNO3 CuCN D E H₂O F Garrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY