
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
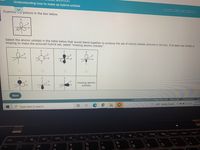
Transcribed Image Text:Understanding how to make sp hybrid orbitals
Examine c picture in the box below.
LITC
Select the atomic orbitals in the table below that would blend together to produce the set of hybrid orbitals pictured in the box. If at least one orbital is
missing to make the pictured hybrid set, select "missing atomic orbitals".
missing atomic
orbitals
Start
O 2021 McGraw Hill LLC. AIl Rights Reserved. Terms of Use Privacy Center AcC
5:48 PM
89°F Mostly cloudy
8/23/202
e Type here to search
End
Insert
B8B
F12
Home
F10
F11
F9
F7
FB
AI
F2
F6
F5
Esc
F3
F4
F1
FnLk
&
$4
%
@
%23
7
8
4
5
6
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Choose the selection which describes an electron-domain geometry and number of pairs of nonbonding electrons located in the valence shell of the central atom which is consistent with a molecule having the molecular geometry shown in the above figure. a) The electron-domain geometry is octahedral. The number of nonbonding pairs is 1.b) The electron-domain geometry is trigonal bipyramidal. The number of nonbonding pairs is 0.c) The electron-domain geometry is trigonal bipyramidal. The number of nonbonding pairs is 2.d) The electron-domain geometry is trigonal bipyramidal. The number of nonbonding pairs is 1.e) The electron-domain geometry is octahedral. The number of nonbonding pairs is 2.arrow_forwardHow is the geometry of small molecules in today's lab determined? Group of answer choices By counting all valence electrons on the central atom. By determining the number of valence elctron domains on the central atom By counting the total number of valence electrons in the molecule.arrow_forwardMatch the atom with how many bonds the atom can make without hybridization. It might help to write out the electron configurations for each atom. Group of answer choices P S Nearrow_forward
- Please help filling chartarrow_forwardGive the hybridization where the arrows point to in the picture attachedarrow_forwardplease please answer super super fast its very important answer before the end of today please answer clear and neat and not messy please please only do the sigma bond drawings and the pi bond drawings draw the sigma and pi bond drawing clearly and bigarrow_forward
- What orbitals are used to form the labeled bonds in the following molecule? Of the labeled C—C bonds, which is the shortest?arrow_forwardChoose the selection which describes an electron-domain geometry and number of pairs of nonbonding electrons located in the valence shell of the central atom which is consistent with a molecule having the molecular geometry shown in the above figure. a) The electron-domain geometry is octahedral. The number of nonbonding pairs is 1. b) The electron-domain geometry is trigonal bipyramidal. The number of nonbonding pairs is 0. c) The electron-domain geometry is trigonal bipyramidal. The number of nonbonding pairs is 2. d) The electron-domain geometry is trigonal bipyramidal. The number of nonbonding pairs is 1. e) The electron-domain geometry is octahedral. The number of nonbonding pairs is 2.arrow_forwarda) Using Valence bond theory, not the VSEPR model, show what orbitals the central carbon atom is using in the compound HCN (No lewis structure). What is the bond angle and molecular geometry in the HCN molecule? b)Draw and name the orbitals for all 3 atoms before and after bonds form.arrow_forward
- Please help, a bit conufsedarrow_forward2) a) Consider the following molecule . Given what you have learned about hybridization theory, draw an image or images explaining the bonding situation in this molecule. I want you to draw out all of the orbitals, hybrid orbitals and how they overlap to form the bonds in the molecule. Indicate the % s or p character in the given atomic and hybrid orbitals. Which C-C bond or bonds are the longest? In a paragraph or so explain the image or images you just drew. b) Lastly, consider the molecule below. Indicate the Molecular formula, the molar mass, label the hybridization of each atom except for hydrogen, indicate any chiral centers with a *, which bond or bonds are the shortest, identify by name of each functional group with an arrow pointing to the group.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY