
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
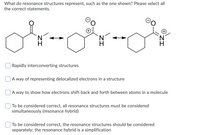
Transcribed Image Text:What do resonance structures represent, such as the one shown? Please select all
the correct statements.
Rapidly interconverting structures
|A way of representing delocalized electrons in a structure
A way to show how electrons shift back and forth between atoms in a molecule
| To be considered correct, all resonance structures must be considered
simultaneously (resonance hybrid)
To be considered correct, the resonance structures should be considered
separately; the resonance hybrid is a simplification
ZI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Using molecular models, construct the following molecules/polyatomic ions, and write their Lewis formulas. Record the following information for each in your laboratory notebook. a) Molecule or ionb) # of valence electronsc) Lewis structured) Draw any resonance structures if applicablee) Calculate all formal charges for molecules that have resonance structuresf) For molecules that have resonance structures identify, which resonance structure contributes the most to the hybrid? 1.PH3 2 HCN 3.PO4 3-arrow_forwardWould you mind to check all the answer on this table because I try to understand the line bond structure and re-draw structure my homework? Could you help me with this question CS2?arrow_forwardWhat is the formal charge on chlorine in the best Lewis structure for chloric acid? type your answer...arrow_forward
- Use Lewis theory to determine the formula for the compound that forms between each of the following pairs of elements. Ca and Te Express your answer as a chemical formula. Mg and Br Express your answer as a chemical formula. Na and S Express your answer as a chemical formula. In and O Express your answer as a chemical formula.arrow_forwardAccording to the VSEPR theory, why are bonds and lone pairs spaces as far apart as possible? Thanks!arrow_forwardMolecule or lon SnCl4 #valence e y **NO3 #valence e 6 **PF6 #valence e 6 SbCl5 #valence e **N3 #valence e Lewis Structure No. of Holes Needed in the Central Atom No. of Regions of Electron Density on Central Atom Electron Geometry Molecular Geometry Sketch Molecule/lon Showing Molecular Geometry (show atoms only!) ** lons are treated in the same way as neutral molecules. (The charge is, of course, used in determining the number of electrons in the valence shell of the ion.)arrow_forward
- Hydrogen cyanide, HCN, is a highly poisonous compound that vaporizes slightly above room temperature. a) How many valence electrons does C have? b) How many valence electrons does H have? c) How many valence electrons does N have? d) How many total valence electrons does the HCN molecule have? e) Draw the Lewis structure for HCN that minimizes the formal charges on all atoms. f) How many electron domains are on the central atom? g) What is the molecular geometry of HCN? h) Is the molecule polar?arrow_forwardConsidering the structure on the far left as the original resonance structure, which of the subsequent resonance structures (A-D) is implausible. Explain why you believe the resonance structure to be implausible.arrow_forward1 What is the total number of valence electrons in the Lewis structure of ICl,"? electrons 2 Draw a Lewis structure for ICl,". • Do not include overall ion charges or formal charges in your drawing. If the species contains oxygen, do not draw double bonds to oxygen unless they are needed in order for the central atom to obey the octet rule. C opy aste Previous Nextarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY