
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
** answer choices for H
singlet
doublet
triplet
quartet
multiplet
**Answer choices for ppm
0.9
2.4
7.2
7.8
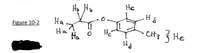
Transcribed Image Text:H. Ho
He
На
Figure 10-2
Ha
Ha
He
·CH3} He
![See Figure 10-2.
Ha would be [ Select ]
at approximately
[ Select ]
ppm.
Hb would be [ Select ]
at approximately [Select]
ppm.
Hc would be [ Select ]
at approximately [ Select ]
ppm.
Hd would be [ Select ]
at approximately [Select]
ppm.
He would be [ Select ]](https://content.bartleby.com/qna-images/question/e6874899-da51-4699-8099-2df04086d4ca/a0543d5e-c1af-4cee-9b70-9576d740b72d/3uiefx9_thumbnail.png)
Transcribed Image Text:See Figure 10-2.
Ha would be [ Select ]
at approximately
[ Select ]
ppm.
Hb would be [ Select ]
at approximately [Select]
ppm.
Hc would be [ Select ]
at approximately [ Select ]
ppm.
Hd would be [ Select ]
at approximately [Select]
ppm.
He would be [ Select ]
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- After creating her standard curve for absorption versus the concentration (M) of FD&C Red 40, a student found that her best fit linear line for FD&C Red 40 was y = 2,962x + 0.005. Her Kool-Aid sample had an absorbance of 0.685. If 0.543 grams of Kool-Aid powder was used to prepare an 8-fl oz cup of her assigned flavor, what is the percent by mass of FD&C Red 40 in her 8-fl oz cup?arrow_forward17) Chemical Formulae: C12H18 8 =0 4H, Doublet Triplet Triplet Doublet elli 18) Chemical Formula: C9H120 IR: Strong broad peak at 3200 cm-¹ PPM 12×2=26-18=-8/2=4 PPM htl=2 Quartet, 2H Singlet, 9H Triplet, 3H 0 0 9x2=18+2=20=12=842arrow_forwardch1arrow_forward
- Identify the detectors in gas chromatography that can be used for the detection of each type of analyte. More than one detector may be appropriate for a given analyte, and a given detector may be appropriate for more than one type of analyte. compounds ionized by UV radiation sulfur or nitrogen containing compounds halogenated compounds flame ionization Answer Bank mass spectrometer nitrogen and phosphorous containing compounds thermal conductivity electrolytic conductivity hydrocarbons electron capture thermionic photoionizationarrow_forwardThe methodology of choice for measuring therapeutic drug levels is Question 4 options: immunoassay chromatography atomic absorption spectrophotometry spectrophotometryarrow_forwardComplete the following table. In the second column, assign each set of molecules as constitutional isomers, conformational isomers, enantiomers, diastereomers, or identical. For the third column, based on that analysis, indicate for each set of molecules if they are separable or inseparable by SiO2 chromatography.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY