
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
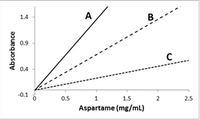
Which of the following methods results in the most sensitive method?
A) All 3 methods have equal sensitivity.
B) The most sensitive method cannot be determined from the information given.
C) B
D) A
E) C
Transcribed Image Text:1.4
B.
0.9
0.4
-0.1
0.5
1.5
2
2.5
Aspartame (mg/mL)
Absorbance
Expert Solution
arrow_forward
Step 1
We are given Absorbance versus concentration of aspartame (mg/mL) graph for 3 different methods A, B and C, and we have to compare their sensitivity.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- A calibration curve was created to determine the quantity of protein in a solution. The calibration curve has the form of a straight line with the equation ?=0.0182?+0.007 where ? is the corrected absorbance of the solution and ? is quantity of protein in the solution in units of micrograms (μg). Determine the quantity of protein in a solution that has an absorbance of 0.305. A blank solution has an absorbance of 0.060. The answer (Quantity of protein) should be in units of (μg)arrow_forwardA solution of a dye was analyzed by spectrophotometry, and the following calibration data were collected using a 1 cm cuvette. *graph in attachemnt* Using the calibration data above for the dye solution, what is the dye concentration in a solution with an absorbance (A) = 0.52 if it was measured in a 1 cm cuvette? Group of answer choices 3.0 x 10-6 M 6.0 x 104 M 2.0 x 10-6 M Not enough information is provided.arrow_forwardcan you explain how I can get 1.596 mg/L of concentration of Fe3+ in dilutes sample and can you also make the calibration curve.arrow_forward
- Q5. Two methods were used to measure fluorescence lifetime of a dye. The average result for Method 1 is = 1.382 ns with a standard deviation of s1 = 0.039 and n1= 7. The average result for Method 2 is = 1.346 ns with a standard deviation of s2 = 0.015 and n2= 5. Is there a difference between the two methods at the 95% confidence level using F test?arrow_forwardNonearrow_forwardSolve both parts otherwise I will downvote ... Need solution asaparrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY