
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
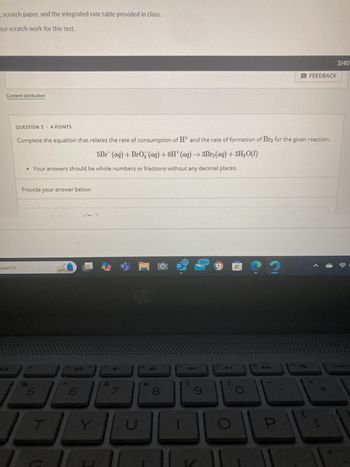
Transcribed Image Text:scratch paper, and the integrated rate table provided in class.
our scratch work for this test.
Content attribution
3/40
FEEDBACK
QUESTION 3 - 4 POINTS
Complete the equation that relates the rate of consumption of H+ and the rate of formation of Br2 for the given reaction.
5Br (aq) + BrO3 (aq) + 6H (aq) →3Br2(aq) + 3H2O(l)
• Your answers should be whole numbers or fractions without any decimal places.
Provide your answer below:
Search
尚
5
fn
40
*
00
99+
2
9
144
a
[
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- Go to the PhET Reactions and change to Angled shot to see the difference. (a) What happens when the angle of the collision is changed? (b) Explain how this is relevant to rate of reaction.arrow_forwardIn the PhET Reactions under Options. (a) Leave the Initial Temperature at the default setting. Observe the reaction. Is the rate of reaction fast or slow? (b) Click Pause" and then Reset All, and then enter 15 molecules of A and 10 molecules of BC once again. Select Show Bonds" under Options. This time, increase the initial temperature until, on the graph, the total average energy line is completely above the potential energy curve. Describe what happens to the reaction.arrow_forward7-10 The rate of disappearance of HCI was measured for the following reaction: The initial concentration of HCI is 1.85 M. Its concentration decreases to 1.58 M in 54.0 min. What is the rate of reaction?arrow_forward
- Iodomethane (CH3I) is a commonly used reagent in organic chemistry. When used properly, this reagent allows chemists to introduce methyl groups in many different useful applications. The chemical does pose a risk as a carcinogen, possibly owing to iodomethanes ability to react with portions of the DNA strand (if they were to come in contact). Consider the following hypothetical initial rates data: [DNA]0 ( mol/L) [CH3I]0 ( mol/L) Initial Rate (mol/Ls) 0.100 0.100 3.20 104 0.100 0.200 6.40 104 0.200 0.200 1.28 103 Which of the following could be a possible mechanism to explain the initial rate data? MechanismIDNA+CH3IDNACH3++IMechanismIICH3ICH3++ISlowDNA+CH3+DNACH3+Fastarrow_forwardDefine stability from both a kinetic and thermodynamic perspective. Give examples to show the differences in these concepts.arrow_forwardThe initial rate for a reaction is equal to the slope of the tangent line at t 0 in a plot of [A] versus time. From calculus, initial rate = d[A]dt . Therefore. the differential rate law for a reaction is Rate = d[A]dt=k[A]n. Assuming you have some calculus in your background, derive the zero-, first-, and second-order integrated rate laws using the differential rate law.arrow_forward
- The acid-catalyzed iodination of acetone CH3COCH3(aq) + I2(aq) CH3COCH2I(aq) + HI(aq) is a common laboratory experiment used in general chemistry courses to teach the method of initial rates. The reaction is followed spectrophotometrically by the disappearance of the color of iodine in the solution. The following data (J. P. Birk and D. L Walters, Journal of Chemical Education, Vol. 69, p. 585, 1992) were collected at 23 C for this reaction. Determine the rate law for this reaction.arrow_forwardGive the Arrhenius equation. Take the natural log of both sides and place this equation in the form of a straight-line equation (y = mx + b). What data would you need and how would you graph those data to get a linear relationship using the Arrhenius equation? What does the slope of the straight line equal? What does the y-intercept equal? What are the units of R in the Arrhenius equation? Explain how if you know the rate constant value at two different temperatures, you can determine the activation energy for the reaction.arrow_forwardFor the reaction of crystal violet with NaOH(aq), the measured rate of reaction is 1.27 106 mol L1 s1 when the concentration of crystal violet cation is 4.13 105 mol/L. (a) Estimate how long it will take for the concentration of crystal violet to drop from 4.30 105 mol/L to 3.96 105 mol/L. (b) Could you use the same method to make an accurate estimate of how long it would take for the concentration of crystal violet to drop from 4.30 105 mol/L to 0.43 105 mol/L? Explain why or why not.arrow_forward
- The rate of the decomposition of hydrogen peroxide, H2O2, depends on the concentration of iodide ion present. The rate of decomposition was measured at constant temperature and pressure for various concentrations of H2O2and of KI. The data appear below. Determine the order of reaction for each substance, write the rate law, and evaluate the rate constant. Rate [H2OJ [Kll (mL min-’) (mol L ’) (mol L ’) 0.090 0.15 0.033 0.178 0.30 0.033 0.184 0.15 0.066arrow_forwardRegular ?ights of supersonic aircraft in the stratosphere ale of concern because such aircraft produce nitric oxide, NO, as a byproduct in the exhaust of their engines. Nitric oxide reacts with ozone, and it has been suggested that this could contribute to depletion of the ozone layer. The reaction NO+O3NO2+O2 is first order with respect to both NO and O3 with a rate constant of 2.20107 L/mol/s. What is the instantaneous rate of disappearance of NO when [NO]=3.3106 M and [O3]=5.9107M?arrow_forwardConsider the zero-, first-, and second-order integrated rate laws. If you have concentration versus time data for some species in a reaction, what plots would you make to prove a reaction is either zero, first, or second order? How would the rate constant, k, be determined from such a plot? What does the y-intercept equal in each plot? When a rate law contains the concentration of two or more species, how can plots be used to determine k and the orders of the species in the rate law?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co