
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:IN +3H, ANH,
7
MILE
In both scenarios depicted graphically,
above, the more abundant reactant is
the limiting reactant.
Cite evidence and reasoning to
explain how this is possible.
Clas
|-|
ỊCH, K2010-20
333333
DS
AAAA
CL
IS
0
225
The more abundant reactant is the limiting reactant because it doesn't
have enough to fulfill the "recipe." In the first scenario, there needs to be
1N2 and 3H2 to make 2NH3. So even though there are 7 H2, only 6 of
those 7 can be made into 2 2NH3, even though the 1 N2 is already enough
to make an NH3 product. This makes H2 the limiting factor even though
it's the most abundant. In the second scenario, there has to be 1 CH4 and
202 to make the products. With that "recipe," 4 CH4 would need 8 02,
but since there are only 6 02, the amount of product that can be made is
limited even though 02 is technically the most abundant reactant.
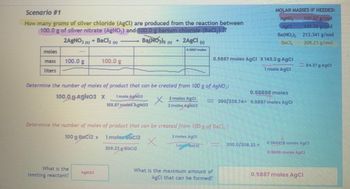
Transcribed Image Text:Scenario #1
How many grams of silver chloride (AgCl) are produced from the reaction between
100.0 g of silver nitrate (AgNO,) and 100.0 g barium chloride (BaCl₂) ??
2AgNO3(s) + BaCl₂ (s)
moles
liters
100.0 g
100 g BaCl2 x
100.0 g
Determine the number of moles of product that can be created from 100 g of AgNO₂:
100.0 g-AgNO3 x
1 mole AgNO3
169.87 moles AgNO3
What is the
limiting reactant?
AgNO3
Ba(NO3)2 (5) + 2AgCl (s)
0.5887 moles
1 moles BaCl2
Determine the number of moles of product that can be created from 100 g of BaCl,:
2 moles AgC!
X
1mol Bach
208.23 g BaCl2
X
2 moles AgCl
2 moles AgNO3
0.5887 moles AgCl X 143.2 g AgCl
1 mole AgCl
MOLAR MASSES IF NEEDED:
169.87 g/mol
143,32 g/mol
213.341 g/mol
208.23 g/mol
0.58868 moles
- 200/333.74= 0.5887 moles AgCl
What is the maximum amount of
AgCl that can be formed?
AgNO.
AgCl
Ba(NO₂)₂
200.0/208.23 =
84.37 g AgCl
0.960476 moles AgCl
0.9606 molos
AgCl
0.5887 moles AgCl
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nitrogen monoxide is produced by combustion in an automobile engine. According to the following reaction, how many grams of oxygen gas are necessary to form 0.842 moles nitrogen dioxide?2NO (g) + O2 (g) 2NO2 (g)arrow_forwardGiven the unbalanced equation, NH4NO2(s) -> N2(g) + H2O(I), how many grams of H2O are produced from the decomposition of 0.42 g of NH4NO2?arrow_forwardNitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: 3 NO: (9) + H2O(1)2 HNO: (1) +NO(9) Suppose that 14 mol NO, and 4 mol H20 combine and react completely. How many moles of the reactant in excess are present after the reaction has completed? O 2 mol H20 O 1 mol NO2 2 mol NO2 O 1 mol H20arrow_forward
- A compound is composed of the elements, carbon, hydrogen, and fluorine only. Combustion of the compound produces CO2, H2O, and HFC?H?F? + O2 ➝ CO2 + H2O + HFCombustion of a 5.000 g sample of the compound produces the products shown in the table below. Product Mass (grams) CO2 8.724 H2O 2.143 HF 2.379 Determine the empirical formula for the compound.arrow_forwardC3H7OH + 9 O2 -----------> 6 CO2 + 8 H2O How many grams of H2O can be produced when 35.00 g of C3H7OH react with 45.00 g of O2?arrow_forwardWhen calcium carbonate reacts with hydrochloric acid, an aqueous solution of calcium chloride, water, and carbon dioxide gas is produced. CaCO3 + 2HCI-CaClna + H,Ony + COte If 0.68 g hydrochloric acid reacts with excess calcium carbonate, what mass of calcium chloride will be produced?arrow_forward
- 3. 4 Al + 3 MnO22 Al2O3 + 3 Mn If 1.528 moles of MnO2 are reacted, how many moles of Al2O3 will be produced?arrow_forwardWhat is the theoretical yield of HCI if 30.0 g of BCI3 and excess H20 are reacted according to the following balanced reaction? BC13(g) +3 H20(1) H3BO3(s) + 3 HCI(g) The following molar masses are: HCI = 36.46 g/mol BCI3 = 117.17 g/mol %3D H20 = 18.02 g/mol 75.9 g HCI 132 g HCI 187 g HCI 56.0 g HCI 28.0 g HCIarrow_forwardAa.84. What is the maximum mass of S8 that can be produced by combining 81.0 g of each reactant? 8SO2 +16H2S⟶3S8 + 16H2Oarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY