
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
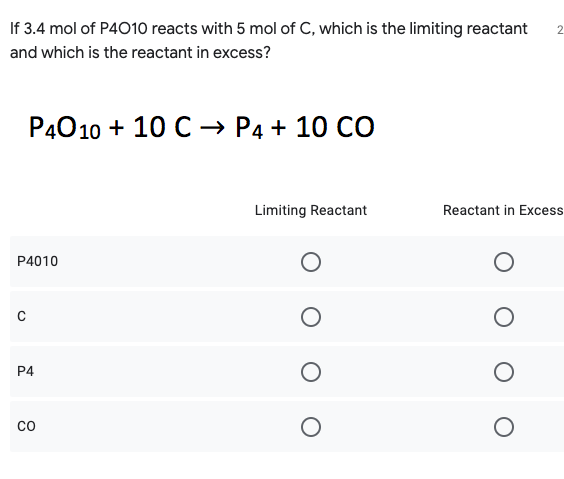
Transcribed Image Text:If 3.4 mol of P4010 reacts with 5 mol of C, which is the limiting reactant
2.
and which is the reactant in excess?
P4010 + 10 C -→ P4 + 10 CO
Limiting Reactant
Reactant in Excess
P4010
P4
co
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 7 images

Knowledge Booster
Similar questions
- Reactant 1 : Fe 0.5g Reactant 2 : S8 1.5g Molecular equation:arrow_forwardBalance each reaction, then suppose exactly 13.3 g of each reactant is taken. Indicate which reactant is the limiting reagent. Calculate the mass of each product that is expected. Al(s) + Cl2(g) → AlCl3(s)limiting reagent AlCl2 AlCl3 producedarrow_forwardConsider the reaction of ammonia as below: 2 NH3(g) + 5 F2(g) N2F4(g) + 6 HF(g) ----> How many moles of ammonia (NH3) are needed to completely react with 13.6 mol of F2? O 34.0 mol 2.27 mol O 6.80 mol O 5.44 molarrow_forward
- Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. The balanced equation for the reaction is:2Mg(s)+O2(g)→2MgO(s)2Mg(s)+O2(g)→2MgO(s) When 10.1 gg of Mg are allowed to react with 10.5 g of O2, 13.4 gg of MgO are collected. Determine the limiting reactant for the reaction. Express your answer as a chemical formula.arrow_forwardIf 100.0 molecules of H2 and 38.0 molecules of O2 react, how many molecules of H2O can be produced in the reaction below? 2 H2(g) + O2(g) → 2 H2O(g) molecules 1 2 4 6. C 7 9. +/- х 100 +arrow_forwardSuppose that 10.0g of each reactant are added to a reaction chamber causing the following reaction: SO2 + NaOH = Na2SO3 + H2O what is the yield of Na2SO3 in grams? Which reactant is in excess? Which reactant is limiting? Which reactant has some left over after the reaction finishes? How much reactant is left overarrow_forward
- For the following unbalanced reaction, if you start with 2.50 mol of N2O(g) how many moles of Ole) can be formed? N2Olg) - N2 (g) + O2 (g) O 5.00 mol O 2.50 mol 1.00 mol O 1.25 molarrow_forwardFor the reaction below, balance the chemical equation and then calculate how many grams of NH3 form when each amount of reactant completely reacts. N2H4 (l) → NH3 (g) + N2 (g) a. 5.3 mol N2H4 b. 32.5 g N2H4 c. 14.7 kg N2H4arrow_forwardA sample of 0.75 g of hydrogen gas was obtained by reacting 32.5 g hydrochloric acid with 18.0 g magnesium. Mg(s) + 2 HCI (aq) --> MgCl₂ (aq) + H₂(g) What is the percent yield for this reaction? 100% 83% 41% 50%arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY