
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Transcribed Image Text:10:58 9
Aa
10:58 AM, Apr 24
Save
Share
II
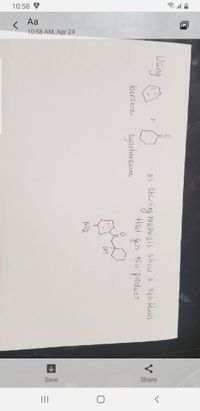
Using
hüterials show &
as Staring hukerials show e syvethesis
that gets this produet
surethusis
benzene
NO2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- what is the reactantarrow_forwardEach pictured Lewis structure is invalid. Identify the error in each case. You are currently in a labeling module. Turn off browse mode or quick nav, Tab to items, Space or Enter to pick up, Tab to move, Space or Enter to drop. Answer Bank octet‑rule violationmulti-use wrong electron totalmulti-usearrow_forwardDraw a structural formula for the major organic product of the reaction shown below. CuLi Consider E/Z stereochemistry of alkenes. • Do not show stereochemistry in other cases. • You do not have to explicitly draw H atoms. • Do not include organocopper or inorganic ion by-products in your answer. C P opy aste ChemDoodlearrow_forward
- Draw the major organic product. Select Draw Rings More C H Br H3C CH3 H3C-C H-Br H. H. |help contact us terms of use about us privacy policy careersarrow_forwardEXP VIDEO https://www.youtube.com/watch?v=9Wxxv7kwXlA&list=PLE-217H0ao60Uzpzj-FNP_0ScVp5SZB7j&index=8 1.Pre lab question What are the functional groups in your substrate? -Nitrate -Nitrate and acetate -Amide and ether -Benzene ring b.The stable reaction intermediate in this reaction is Nitronium ion Benzonium ion Carbocation Carbanion c.How would you distinguish between 2-nitrophenacetin and 3-nitrophenacetin? -Color difference -Melting point difference -Difference in Molecular weight -They cannot be differentiated. dAn ether group is -Ortho para activating -Meta activating -Ortho para deactivating -ring activator eThe catalyst in this experiment was -Glacial acetic acid -Nitric acid -Sulfuric acid -Ethanol fThe chemical waste from this experiment should be discarded in the -Sink -Halogenated waste container -non-halogenated waste container -Heavy metal waste container. gWhat is the limiting reagent in this experiment? -Nitric acid -Glacial acetic acid -Phenacetin…arrow_forward-IES.pat Tamaki-Amajiki | De... Popular and Irendi... New lab https://www.gillitv. Sa Re ensation - Drawing i Saved Determine the product formed when butanone (CH CH,COCH2) Is treated with each reagent. If no reaction occurs, label the reaction with "no reaction". PCC H2 Pd-C но он Na,Cr;O7 no reaction [1] LIAIH, [2] H,0 Ag,0 NH,OH NaBH, CH,OH Reset Prev 1 of 11 Next >arrow_forward
- MAIN QUESTION Acyl transfer: ester saponification Question 1 of 5 Write the first step of the reaction mechanism using curved arrows to show electron reorganization. Electron pairs ERASE H₂C-C CH3 OCH3 - NaOH H₂O -H₂C-00 :O: || C | :O-CH3 O Na H-O-CH3 I H :O: H-O- -H I Na + Submitarrow_forwardHow are these products being formed? Show steps and arrowsarrow_forwardAcceptable Mechanison? - Œ 50% a I Acceptable Mechanismy- Ph-= -Pr 10215, CH3CH2NH₂ ZonMy Go (31)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY