
Fundamentals Of Analytical Chemistry
9th Edition
ISBN: 9781285640686
Author: Skoog
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Question
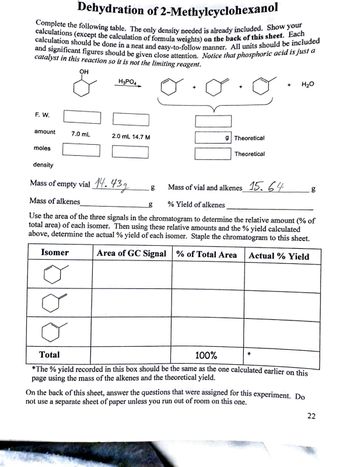
Transcribed Image Text:Dehydration of 2-Methylcyclohexanol
Complete the following table. The only density needed is already included. Show your
calculation should be done in a neat and easy-to-follow manner. All units should be included
calculations (except the calculation of formula weights) on the back of this sheet. Each
and significant figures should be given close attention. Notice that phosphoric acid is just a
catalyst in this reaction so it is not the limiting reagent.
OH
F. W.
H3PO4
amount
7.0 mL
2.0 mL 14.7 M
g Theoretical
moles
Theoretical
+ H₂O
density
Mass of
empty vial
14.43g
g
CD
Mass of vial and alkenes 15.64
g
Mass of alkenes
g
% Yield of alkenes
Use the area of the three signals in the chromatogram to determine the relative amount (% of
total area) of each isomer. Then using these relative amounts and the % yield calculated
above, determine the actual % yield of each isomer. Staple the chromatogram to this sheet.
Isomer
Area of GC Signal % of Total Area Actual % Yield
Total
100%
*
*The % yield recorded in this box should be the same as the one calculated earlier on this
page using the mass of the alkenes and the theoretical yield.
On the back of this sheet, answer the questions that were assigned for this experiment. Do
not use a separate sheet of paper unless you run out of room on this one.
22
![Sample Name: Test2
Acq. Operator
: SYSTEM
Sample Operator: SYSTEM
Acq. Instrument : 6890N_GC-FID
Injection Date
Acq. Method
Last changed
: 11/11/2024 2:21:09 PM
Location :
1
Inj Volume Manually
: C:\CHEM32\1\METHODS \DEHYD351L.M
: 11/8/2024 3:26:55 PM by SYSTEM
Analysis Method : C:\CHEM32\1\METHODS\DEF_GC.M
11/12/2024 10:20:57 AM by SYSTEM
FID1A, (CHEM351LITest2 2024-11-11 14-21-09.D)
Last changed
PA
1750
1500
1250
1000
750
500
250
0
1.226
1.338
1.393
1.445
1.513
0.2
0.4
0.6
0.8
1.2
1.4
1.6
1.8
min
Area Percent Report
Sorted By
Multiplier
:
Signal
1.0000
Dilution
1.0000
Use Multiplier & Dilution Factor with ISTDS
# [min]
Signal 1: FID1 A,
Peak RetTime Type Width
----|-------|----|-------|-------|
Area
[min] [PA*s]
Height
[PA]
Area
%
|--------|
1 1.226 BB
0.0138
2.49083
2.96068 0.12408
2
1.338 BB 0.0173
6.06945
5.33509 0.30236
3
1.393 BV
0.0175
180.39203
156.04135
8.98645
4
1.445 VB
0.0147
17.87214
5
1.513 BB S
19.50680 0.89032.
0.0151 1800.55408 1899.02905 89.69679
Totals:
2007.37852 2082.87298
6890N_GC-FID 11/12/2024 10:26:38 AM SYSTEM
Page
1 of 2](https://content.bartleby.com/qna-images/question/aa15ffa4-06ff-402c-ae2f-dda95408fa6d/1fd7a3e5-ec5f-4a2a-8651-964c177fa6a0/yugi1d6_thumbnail.jpeg)
Transcribed Image Text:Sample Name: Test2
Acq. Operator
: SYSTEM
Sample Operator: SYSTEM
Acq. Instrument : 6890N_GC-FID
Injection Date
Acq. Method
Last changed
: 11/11/2024 2:21:09 PM
Location :
1
Inj Volume Manually
: C:\CHEM32\1\METHODS \DEHYD351L.M
: 11/8/2024 3:26:55 PM by SYSTEM
Analysis Method : C:\CHEM32\1\METHODS\DEF_GC.M
11/12/2024 10:20:57 AM by SYSTEM
FID1A, (CHEM351LITest2 2024-11-11 14-21-09.D)
Last changed
PA
1750
1500
1250
1000
750
500
250
0
1.226
1.338
1.393
1.445
1.513
0.2
0.4
0.6
0.8
1.2
1.4
1.6
1.8
min
Area Percent Report
Sorted By
Multiplier
:
Signal
1.0000
Dilution
1.0000
Use Multiplier & Dilution Factor with ISTDS
# [min]
Signal 1: FID1 A,
Peak RetTime Type Width
----|-------|----|-------|-------|
Area
[min] [PA*s]
Height
[PA]
Area
%
|--------|
1 1.226 BB
0.0138
2.49083
2.96068 0.12408
2
1.338 BB 0.0173
6.06945
5.33509 0.30236
3
1.393 BV
0.0175
180.39203
156.04135
8.98645
4
1.445 VB
0.0147
17.87214
5
1.513 BB S
19.50680 0.89032.
0.0151 1800.55408 1899.02905 89.69679
Totals:
2007.37852 2082.87298
6890N_GC-FID 11/12/2024 10:26:38 AM SYSTEM
Page
1 of 2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Sample IdentificationCodeConcentration of M%TA=2-log(%T)Q50004.00 x 10-417.90.75Q50013.20 x 10-425.00.6Q50022.40 x 10-435.70.46Q50031.60 x 10-450.20.3Q50048.000 x 10-570.80.15SampleIdentificationCode%TA=2-log(%T)AMQ0210150143.70.359518560.360.000192Q0210150244.10.355561410.360.00018Q0210150343.80.358525890.360.00017Q0210150444.10.355561410.360.00018Q0210150543.80.358525890.360.00017What was their percent error?43%Does Batch 021015 meet legal requirements?No, because it is not between 2.85 * 10(4) and 3.15 * 10(4)Well #DropsBluedye1234567891012345678910Drops 9Distilled water876543210Concne 0.26tration0.52Test Tube #0.781.041.3Solutions3Concentration (M)2.082.32.6Concentration (ppm)1:1 dilution11.82Starting Dilution21.562:1 dilution0A.Zero standard0Was your calibration curve as linear as you expected?B.Did you experience any “drift†of the resistance readings?C.What is the equation of your best-fit line?D.What commercial drink did you analyze?E.Assuming…arrow_forwardS outo Classes e myBackpack C Infinite Campus 9 New Tab File| /media/fuse/drivefs-a644cf067c63cc0d1217e47dfd368497/root/k.g_k.sch_ZG9taW5pY28ua2FudG9yQGFwc2sxMi5vcmc_Whiteboar. Q < * 1 /1 Good Day 03 Be O +2 AIC→I AbO, •3 Be Cla a) what is the molar mass? BeO , AlzOa b) How many mols of beryllium chleride are produced from 13 mols of BeO?(1-step) ) How many grams of Al,O, are 9 mol AICI,? (2-step) ) How many grams of Al2Og are produced Srom 100g BeO (3-step) e) Determine the limit ing reartan ti 13 7mol BeO ;13.7mol Al Cl, produced from 5) Determine excess reactant 4Sa BrO j 75q AICIS US VO 11:12 @ %23 2$ % 4 7 8 backsp W e y d h karrow_forwardSrCl, Observations B|I|U X X + fx | ®| D e Normal Activated charcoal observations Normal BIU fx| 田=| || 的|| || In 1. Based on your understanding of color's relationship to wavelength, identify the approximate wavelength of light (nm) emitted by strontium when it was burned. (No quantitative data was collected for this; you are giving an approximate value only, based on what you observed.) Explain your reasoning in full, making sure to cite specific data and observations to support your answer. Normal BIIIU X:| X'|- EE = fx| DI e 2. While boiling potatoes for dinner in salted water, the pot boils over and you notice that the flame on your gas burner turns to bright yellow-orange. How would you explain the appearance of a color in the previously blue flame? Normal BIIU X X + 田=|IT| 3. What would you conclude about chloride based on your results in the flame tests? Xe | X° | → EI fx| fr DI e E E IT E IT Normal BIIU 4. Place the metal solutions you tested in order of increasing energy…arrow_forward
- 3 Micros X 6 Modul X I Course X S What i Te219 x G intelle x Intelle x com/course.html?courseld%=16985674&OpenVellumHMAC=a46387336edc299b16 49c591bf9ad36#10001 Provide Feedback 11:18 AM 75°F Partly sunny O O 4x a 12/10/2021 36arrow_forwardM9. Readings * OWLV2 | Assig X MindTap - Cen x g.cengage.com/static/nb/ui/evo/index.html?elSBN=9781337790840&id%3D299783426&snapshotld-785765& MindTap- Cen x G amu of ca-G x b My Question x + CENGAGE MINDTAP Chapter 8: Chemical Composition L. E AA Tools Problems 45. Calculate the percent by mass of each element in the following compounds. a. HC103 b. UF4 с. СаН2 d. Ag,S e. NaHSO3 f. MnO2arrow_forwardW Practice 4 - CHEM 1201 co Course: 2020 Spring CHEM b My Questions | bartleby A https://www.webassign.net/web/Student/Assignment-Responses/randomize?pos=0&dep=22671454&tags=autosave#question359495_0 ... TurmuJIgmmy you JuDTunoweTay qucJuon. TUuurc Chapters 9 & 10 required to use a new randomization after every 1 question submissions. O Description Assignment Scoring Your last submission is used for your score. 1. 0/0 POINTS PREVIOUS ANSWERS 2/100 Submissions Used MY NOTES ASK YOUR TEACHER Choose the correct total number of electron domains (bonding and nonbonding) about a central atom if the angle(s) between the electron domains have two different values, either about 90 or 180 degrees. O a) The central atom has 4 electron domains. O b) The central atom has 6 electron domains. O c) The central atom has 3 tron domains. O d) The central atom has 2 electron domains. O e) The central atom has 5 electron domains. 2. 0/0 POINTS PREVIOUS ANSWERS 5/100 Submissions Used MY NOTES ASK YOUR…arrow_forward
- Chrome File Edit View History Bookmarks Profiles Tab Window Help Mon Feb 21 7:26 PM 22S-ENGL110-059: Seminar in x Question 24 of 34 - Homework X group 17 (VIIA), period 5 : - Go X b Chrome File Edit View History E X assessments.macmillanlearning.com/sac/5343243#/5343243/23/-1 Update : O Assignment Score: Lx Give Up? O Hint Check Answer 82.4% Resources Question 24 of 34 > Give the nuclear symbol (isotope symbol) for the isotope of bromine, Br, that contains 46 neutrons per atom. Replace the question marks with the proper integers. nuclear symbol: Br NURS120 Chapt...pptx Show All FEB 3 CC 21 étv P 280 PNG !!arrow_forwardBio 304 H XQ mastering chemistry - Search x Ô https://openvellum.ecollege.com/course.html?courseld=17485264&OpenVellumHMAC-c9157316bcac6b54948b744e0b0e6b4f#10001 folder M Reading Mode: 1.5... M Gmail Course Homearrow_forwardfill in the missingarrow_forward
- n 1. Record da Table 1. Data for time and temperature. Note that the gray areas are where the experimental data is recorded. The other columns are calculated. Exp 1 2 3 Time Seconds 1/time 151.8 0.0066 S 50s 126.6 S In (1/time) (Will be negative) -5.02 -3.91 0.02 0.0079 -4.84 °C K 40 1/K (will be small) 16.5 289.65 0.0035 313.15 0.0032 26 299.15 0.0033 2. Use the data on Table 1 to construct a linear y versus x graph of In(1/time) versus 1/temperature(K) in Excel. The graph should have a title that indicates it is a plot of data for the linear form of the Arrhenius equation. Each axis should have a named quantity label and x-axis should additionally have a unit label. Attach to this graph to the report page. 3. State the slope(with correct units and sign) of the line on the graph constructed in #2 4. Use the stated slope in #3 to determine the energy of activation in kilojoules per mol. Show your work. 5. Using the trendline equation for your graph, predict the time that the reaction…arrow_forwardEgwuekwe-Ou x * Office Editing for Docs, Sheets & Slides| chrome-extension://bpmcpldpdmajfigpchkicefoigmkfalc/views/app.html aboutblank ALL2022 (1).doc Format Tools Help ~ HQ Q Normal Unsaved change 12 M HCI should we use? a. 3 L b. 1 L c. 2 L Edits will not be automatically saved. Times New ... e 28. 4 M solution of NaOH means a. 4 moles of NaOH in 1 L b. 4 grams of NaOH in 1L c. 4 moles of NaOH in 100 mls about:blank 31. What is a catalyst? 27. We have 12 M HCl and need 2 L of 6 M HCl. How many Liters of 30. Equilibrium is the state in which 12 ▾ X W FINALFALL2022 (1).doc Save now BIU A A 29. A gas mixture of N₂, O₂ and He exerts a total pressure of 1 atmosphere. N₂ exerts a pressure of 0.2 atmospheres. O₂ exerts a pressure of 0.5 atmospheres. What is the pressure of He? a. The rate of the forward reaction is greater than the reverse reaction. b. The rate of the reverse reaction is greater than the forward reaction. c. The rate of the forward reaction and reverse reaction are equal.…arrow_forwardplease answer the following with equations used and calculations.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning
