
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
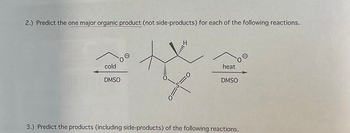
Transcribed Image Text:2.) Predict the one major organic product (not side-products) for each of the following reactions.
cold
DMSO
0=
S:
heat
DMSO
3.) Predict the products (including side-products) of the following reactions.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Show how you would synthesize the following compounds from alkyl halides, vinyl halides, and aryl halides containing no more than six carbon atoms. Part C [1] A Br CuLi Draw the missing molecule on the canvas by choosing buttons from the Tools (for bonds and charges), Atoms, and Templates toolbars. ΔΕ Submit Li Cul Request Answer H: 120 EXP CONT L Marvin JS d by ChemAxon ? DA i..i **** H C N O S CI Br I P F TIarrow_forward4. Reactions, Pushing Electrons, and Thermodynamics Reaction 1: (a) o Draw an arrow pushing mechanism for the elimination reaction below and draw all products formed (including all organic and inorganic products). 'Bu = tert-butyl. (b) ( HO H H H&C..Bu H3C Br In going from reactants to products, does charge stability increase or decrease? (c) Is the reaction reversible (yes or no answer) at room temperature (i.e., not adding heat energy)?arrow_forward10. What is the major organic product generated in the reaction below? HO HO 40||!!!!!! (A) OH ...... (B) 1) 03 2) (CH3)2S HO O (C) H НО. (D)arrow_forward
- ugen.wileyplus.com/edugen/student/ aintr.uni Klein, Organic Chemistry, 3e actice Assignment Gradebook ORION Downloadable eTextbook gnment Give the major organic product of the following reaction. :O + HN (CH3)2CHI H30+ ? heat OH y.arrow_forwardDetermine the major organic products for the reactions in addition to whether they are racemic or not?arrow_forward4. Protonation of alcohol A and subsequent loss of water, produces the intermediate B. CH3 CH; CH, CH, — с — сH, CH, CH;CH, - C- CH, CH; | OH A B (a) (i) Name alcohol A. (11) What type of species is intermediate B? (iii) Draw the structures of the two alkenes which can be formed from species B by removal of a proton. Label as C the alkene which shows geometrical isomerism. (b) The intermediate B is readily attacked by nucleophiles such as water. What is the essential feature of a nucleophile? (c) State what final colour you would see if alcohol A were warmed with acidified potassium dichromate(VI). Explain your answer. Colour Explanation . (ii) Draw two structural isomers of alcohol A which form branched chain ketones when heated with acidified potassium dichromate(VI), but which could not form alkene C on dehydration.arrow_forward
- 1. Using only reactions we have discussed in class, fill in the missing intermediates(two), regents(two), and the fill product in the following multi step synthesis. Assume all reactions proceed too quickly for rearrangements to take place/arrow_forwardWhich of the following methods can be used to synthesize 2-methyl-1-hexene, shown below, with no formation of isomeric by-products? ОН H2SO4 A) heat H2SO4 OH B) heat Ci (CH3);CO Na* + D) H2C=P(C,H5);arrow_forwardDraw the structure(s) of the major organic product(s) of the following reaction. 1. Dry Et₂O + CH3Mgl H 2. aqueous HCI at 0° • You do not have to consider stereochemistry. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu. + √n [ ? ChemDoodlearrow_forward
- In the light-catalyzed alkane chlorination mechanism, the first stage (first step) consists of the cleavage t DY -C-H »r ܘܘ: ܘܘ: ܀ X- + :Q -:+ *@ ܘ: ܘ: :ܘ: ܘ:arrow_forwardSubstitution and elimination: predict the product Maximum allowed tries per question: Unlimited (7) Draw the major organic product of the reaction. Follow this procedure: determine whether the reaction conditions are acidic or basic; identify the most nucleophilic/basic atom, the electrophilic atom, and the leaving group; predict whether elimination or substitution will occur; and then draw the product. Indicate the stereochemistry at every stereocenter with a single wedged (up), hashed (down), or wavy (a mixture of up and down; either) bond. Launch MarvinJSTM viewer or click image to copy source CH3 H3C EtONa EtOH Brarrow_forwardWhat is the product of the following electrocyclic reaction? heat Oa. Ob. エarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning