
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN: 9781305580343
Author: Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
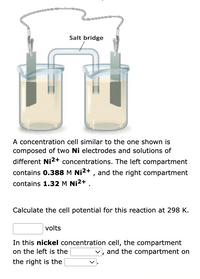
Transcribed Image Text:Salt bridge
A concentration cell similar to the one shown is
composed of two Ni electrodes and solutions of
different Ni2+ concentrations. The left compartment
contains 0.388 M Ni2+ , and the right compartment
contains 1.32 M Ni2+ .
Calculate the cell potential for this reaction at 298 K.
volts
In this nickel concentration cell, the compartment
on the left is the
and the compartment on
the right is the
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider a galvanic cell for which the anode reaction is 3 Pb(s)Pb2+(1.0102M)+2e and the cathode reaction is VO2+(0.10M)+2H3O+(0.10M)+eV3+(1.0105M)+3H2O(l) The measured cell potential is 0.640 V. Calculate E for the VO2+V3+ half-reaction, usingE(Pb2+Pb) from Appendix E. Calculate the equilibrium constant (K) at 25°C for thereaction Pb(s)+2VO2+(aq)+4H3O+(aq)Pb2+(aq)+2V3+(aq)+6H2O(l)arrow_forwardDetermine the overall reaction and its standard cell potential at 25 C for the reaction involving the galvanic cell made from a half-cell consisting of a silver electrode in 1 M silver nitrate solution and a half-cell consisting of a zinc electrode in 1 M zinc nitrate. Is the reaction spontaneous at standard conditions?arrow_forwardAn aqueous solution of an unknown salt of gold is electrolyzed by a current of 2.75 amps for 3.39 hours. The electroplating is carried out with an efficiency of 93.0%, resulting in a deposit of 21.221 g of gold. a How many faradays are required to deposit the gold? b What is the charge on the gold ions (based on your calculations)?arrow_forward
- A galvanic cell is constructed in which the overall reactionis Cr2O72(aq)+14H2O+(aq)+6I(aq)2Cr3+(aq)+3I2(s)+21H2O(l) Calculate E for this cell. At pH 0, with [Cr2O72]=1.5M and [I]=0.40M, the cell potential is found to equal 0.87 V. Calculatethe concentration of Cr3+(aq) in the cell.arrow_forwardCalculate the cell potential of a cell operating with the following reaction at 25C, in which [Cr2O32] = 0.020 M, [I] = 0.015 M, [Cr3+] = 0.40 M, and [H+] = 0.60 M. Cr2O72(aq)+6I(aq)+14H+(aq)2Cr3+(aq)+3I2(s)+7H2O(l)arrow_forwardConsider the following galvanic cell: A 15 0-mole sample of NH is added to the Ag compartment (assume 1.00 L of total solution after the addition). The silver ion reacts with ammonia to form complex ions as shown: Ag+(aq)+NH3(aq)AgNH3+(aq)K1=2.1103AgNH3+(aq)+NH3(aq)Ag(NH3)2+(aq)K2=8.2103 Calculate the cell potential after the addition of 15.0 moles of NH3.arrow_forward
- The Nernst equation allows determination of the cell potential for a galvanic cell at nonstandard conditions. Write out the Nernst equation. What are nonstandard conditions? What do , n, and Q stand for in the Nernst equation? What does the Nernst equation reduce to when a redox reaction is at equilibrium? What are the signs of G and when K 1? When K 1? When K = 1? Explain the following statement: determines spontaneity, while determines the equilibrium position. Under what conditions can you use to predict spontaneity?arrow_forwardDetermine the standard cell potential and the cell potential under the stated conditions for the electrochemical reactions described here. State whether each is spontaneous or nonspontaueous under each set of conditions at 293.15 K. (a) Hg(l)+S2(aq,0.10M)+2Ag+(aq,0.25M)2Ag(s)+HgS(s) (b) The galvanic cell made from a half-cell consisting of an aluminum electrode in 0.015 M aluminum nitrate solution and a half—cell consisting of a nickel electrode in 025 M nickel(l) nitrate solution. (c) The cell made of a half-cell in which 1.0 M aqueous bromide is oxidized to 0.11 M bromine ion and a half-cell in which aluminum ion at 0.023 M is reduced to aluminum metal. Assume the standard reduction potential for Br2(l) is the same as that of Br2(aq).arrow_forwarda Calculate G for the following cell reaction: Tl(s)Tl+(aq)Pb2+(aq)Pb(s) The Gf for Tl+(aq) is 32.4 kJ/mol. b From G, calculate the standard cell potential for the cell reaction and from this, determine the standard potential for Tl2+(aq)+eTl(s).arrow_forward
- In the electrolysis of a solution containing Ag+(aq), metallic Ag(s) deposits on the cathode. Using a current of 1.12 A for 2.40 hours, what mass of silver forms?arrow_forwardCalculate the cell potential of a cell operating with the following reaction at 25C, in which [MnO4] = 0.010 M, [Br] = 0.010 M. [Mn2] = 0.15 M, and [H] = 1.0 M. 2MNO4(aq)+10Br(aq)+16H+(aq)2MN2(aq)+5Br2(l)+8H2O(l)arrow_forwardThe cell potential of the following cell at 25C is 0.480 V. ZnZn2+(1M)H+(testsolution)H2(1atm)Pt What is the pH of the test solution?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks ColeChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks ColeChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemical Principles in the Laboratory
Chemistry
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Brooks Cole

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co