
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Sane
Resources Feedback
Meume
EQuestion 32 of as>
O Attempt 5
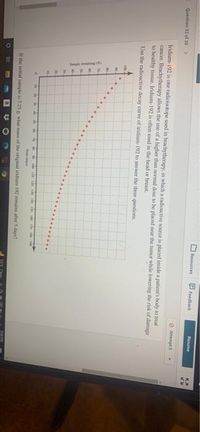
Iridium-192 is one radioisotope used in brachytherapy, in which a radicactive source is placed inside a putient's body to treat
cancer. Brachytherapy allows the use of a higher than normal dose to be placed near the tumor while kowering the risk of damage
to healthy tissue. Iridium-192 is often used in the head or breast.
Use the radioactive decay curve of iridium-192 to answer the three questions
NO.
ia e e
Ti
It the initial sample is 7.25 , what mass of the original iridium 192 remains afher 5 days?

Transcribed Image Text:Resources D Feedback
Resume
of a5>
Attempts
Iridium-192 is one radioisotope used in brachytherapy, in which a radioactive source is placed inside a patientrs body to treat
cancer. Brachytherapy allows the use of a higher than normal dose to be placed near the tumor while kowering the risk of damage
to healthy tissue. Iridium-192 is often used in the head or breast.
Use the radioactive decay curve of iridium- 192 to answer the three questions
Ti
It the initial sample is 7.25 , what mass of the original iridium 192 remains afher 5 days?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I Review I Constants I Periodic Table Every radioactive isotope decays at a certain rate. It is never known when a given nucleus will decay, but if many nuclei are gathered together, as a group they will decay at a measurable and consistent rate. A radioactive Part A half-life is the time it will take for half of the nuclei to Phosphorus-32 has a half-life of 14.0 days. Starting with 8.00 g of 32P, how many grams will remain after 98.0 days ? decay. Express your answer numerically in grams. • View Available Hint(s) ? Submit Part B Carbon-14 has a half-life of 5730 years. In a plant fossil, you find that the 14C has decayed to 1/4.00 of the original amount. How long ago was this plant alive? Express your answer numerically in years. View Available Hint(s) yеars Submitarrow_forwardim a student plz help w this question.arrow_forwardMass defect and binding energy of potassium 39arrow_forward
- If 2.7 * 10−10% of the atoms of a radioactive isotope disintegrate in 1.0 yr, what is the decay constant of the process? Enter your answer in scientific notation.arrow_forwardHow many days will it take for 88Ra with half life of 15 days decay from 2 mole to 0.5 molearrow_forwardThe radioisotope Cerium-141 is used to assess blood flow through the heart in patients. It is a beta emitter. Please use the text box below to write out or add an image of the resulting daughter nucleus that is produced: 141, (Cerium-141 = ¹4Ce) 58arrow_forward
- n 14 of 20 Iridium-192 is one radioisotope used in brachytherapy, in which a radioactive source is placed inside a patient's body to treat cancer. Brachytherapy allows the use of a higher than normal dose to be placed near the tumor while lowering the risk of damage to healthy tissue. Iridium-192 is often used in the head or breast. Use the radioactive decay curve of iridium-192 to answer the three questions. 100 90 80 - 70- 60 - 50 - 40- 30 20 10 10 20 30 40 50 60 70 80 90 100 110 120 130 140 150 160 170 180 190 Time (days) If the initial sample is 2.50 g, what mass of the original iridium-192 remains after 5 days? Sample remaining (%)arrow_forwardFocus on the second nuclear equation shown below (vanadium on the left side of the arrow, titanium and a blank on the right). To balance the bottom numbers (total electric charge) on both sides of the arrow, the charge of the blank must be Symbol(s) Nuclear Equation fill in the gaps Name Representation Description S'v→ Cr+B "Ge Ga+e* 70 He or fa (High-energy) helium nuclei consisting of two protons and two neutrons Alpha particle 24 fe or B V→Ti+ "C→"N + Beta particle (High-energy) electrons fe or B Particles with the same mass as an electron but with 1 unit of positive charge Positron 105 105 50 Sn + 92 IH or tp Proton Nuciei of hydrogen atoms 2Th Th+y 20 Po Pb+ a 234 Particles with a mass approximately equal to that of a proton but with no charge Neutronarrow_forwardQ3 Radium-224 decays through the pathway a, a, a, ß, a, ß. What intermediates are formed in this decay series? What is the final stable nucleus formed?arrow_forward
- I need help balancing these nuclear equations. Thank youarrow_forward18. Samarium-151 is a beta emitter enba ue aUM the product nucleus. 19. How much of a 24-gram sample of Radium-226 will remain unchanged at the end of three half-life periods? Accessibility: Good to go a iparrow_forwardUse the References to access important values if needed for this question. Fill in the nuclide symbol for the missing particle in the following nuclear equation. e+Po 214P. 84 0. Submit Answer Retry Entire Group 4 more group attempts remainingarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY