
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
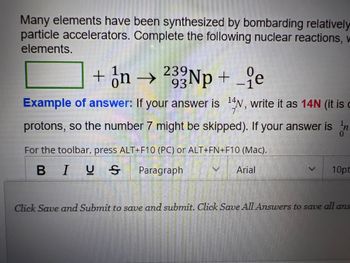
Transcribed Image Text:Many elements have been synthesized by bombarding relatively
particle accelerators. Complete the following nuclear reactions, w
elements.
+ n → 233Np + _e
93
Example of answer: If your answer is 14N, write it as 14N (it is
protons, so the number 7 might be skipped). If your answer is n
For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac).
BIUS Paragraph
Arial
V 10pt
Click Save and Submit to save and submit. Click Save All Answers to save all ans
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the complete decay equation for the given nuclide in the complete A ZXN notation. Refer to the periodic table for values of Z. (Use 01β to signify the following part of the equation: ?+ + ?e.) ?+ decay of 115Tearrow_forwardWrite the complete decay equation for the given nuclide in the complete X notation. Refer to the periodic table for values of Z. Electron capture by 75Se chemPad Help Oreak- Sete' - BAr+e+D_ 75 34Sete75 33A+"0_+le+D Your answer contains improper superscript or subscript formatting. Write the complete decay equation in the X notation. Refer to the periodic table for values of Z. (Use to signify the following part of the equation: B decay producing 1378a. The parent nuclide is a major waste product of reactors and has chemistry similar to potassium and sodium, resulting in its concentration in your cells of ingested. chemiad Help Greek 6881 +B+ve 137_560s 82->227 560 810-beta Your answer contains improper superscript or subscript formattingarrow_forwardq eq creq 2req 2req 2req 3 # E D Fill in the nuclide symbol for the missing particle in the following nuclear equation. He+310T1 Q C Submit Answer $4 R F V Retry Entire Group 9 more group attempts remaining % 5 [Review Topics] [References] Use the References to access important values if needed for this question. T Cengage Learning Cengage Technical Support G ^ 6 R MacBook Pro Y H & 7 N U * 00 8 J LE 1 M ( 9 K A O V ) C ** L P A Previous Email Instructor > + 11 { = [ Next> Save and E 11 ? #arrow_forward
- Refer attached picture. Kindly check the answer in picture containing the question before submitting the solution.arrow_forwardComplete and balance each of the given nuclear equations by supplying the missing particle. 100 Fm 247E. 49 Mn 25 SO Sn 1266 Sb + contact us help about us careera terms of use X 贝arrow_forwardPart 1 of 2 Calculate the energy released when a Bi-210 isotope decays to Tl-206. Round your answer to 3 significant digits. Particle Mass (amu) Bi-210 209.98412 T1-206 205.97611 He-4 4.002603 Note: Reference the Fundamental Constants table for additional information. AE= J x10arrow_forward
- missing particles and coefficientsarrow_forwardSee attached imagearrow_forward1:39 Thu May 9 Done AA ⚫ prod02-cnow-owl.cengagenow.com Chapter 11 Homework [References] Question 1 1 pt Question 2 1 pt Question 3 O 1 pt Question 4 1 pt In the Po-218 natural decay series, the Po-218 initially undergoes a decay, the resulting daughter emits a ẞ particle, and the succeeding daughters emit a a decay series. and ẞ particle in that order. Determine the product of each step in the Po-218 Enter the symbol, mass number and atomic number of each product. (Express your answer as an isotope.) Question 5 1 pt Step 1: a decay Question 6 1 pt Step 2: ẞ decay Question 7 1 pt Step 3: a decay Question 8 1 pt Question 9 1 pt Step 4: ẞ decay Question 101 pt Submit Answer Try Another Version 3 item attempts remaining Question 11 1 pt Question 12 1 pt Question 13 1 pt Question 14 1pt Question 151 pt Question 16 1 pt Question 17 1 pt Question 18 1 pt Question 19 1 pt Question 20 1 pt Visited ≈ 21% 0arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY