
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
A group of students enrolled in a
answer 1,2,3
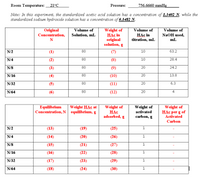
Transcribed Image Text:Room Temperature:
21°C
Pressure:
756.6660 mmHg
Note: In this experiment, the standardized acetic acid solution has a concentration of 1.3402 N, while the
standardized sodium hydroxide solution has a concentration of 0.1482 N.
Weight of
HẠc in
original
solution, g
Original
Concentration,
Volume of
Volume of
Volume of
НАс in
titration, mL
NaOH used,
mL
Solution, mL
N
N/2
(1)
80
(7)
10
63.2
N/4
(2)
80
(8)
10
28.4
N/8
(3)
80
(9)
20
24.2
N/16
(4)
80
(10)
20
13.8
N/32
(5)
80
(11)
20
6.3
N/64
80
(12)
20
4
Weight of
НАС
adsorbed, g
Weight of
HAc per g of
Weight of
Equilibrium
Concentration, N equilibrium, g
Weight HAc at
activated
carbon, g
Activated
Carbon
N/2
(13)
(19)
(25)
1
N/4
(14)
(20)
(26)
1
N/8
(15)
(21)
(27)
N/16
(16)
(22)
(28)
1
N/32
(17)
(23)
(29)
N/64
(18)
(24)
(30)
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- the Question 5 A graph that displays a straight line when the reciprocal of the concentration is plotted as a function of time. The graph has a slope of 0.176 and a y-intercept of 0.837. What is the concentration? (the answer should be entered with 3 significant figures; do not enter units; give answer in normal notation- examples include 1.23 and 12.3 and 120. and -123) Selected Answer O 5.68 Correct Anwer O 1.19 196arrow_forwardWhat is the measurementarrow_forwardNow it's time to create, analyze and interpret a Chemistry-based graph! Let's do this in steps. Data Measurement number Temperature (°C) Solubility (g NaCl/100 g water) 1 0.00 35.2 2 20.0 36.1 3 40.0 37.0 4 60.0 37.7 5 80.0 38.4 100.0 39.0 Let's assume that you just finished your first research laboratory experiment and you collected the data listed above. Pay close attention to the titles. Based on the titles listed answer and complete the following: a. What would be the title of the graph? b. What set of data would you place on the y-axis? This is your dependent variable-measurable variable whose value depends on the independent variable. c. What set of data would you place on the x-axis? This is your independent variable-measurable variable of which when changed, the value of the dependent variable changes. d. Based on the data listed above, use the following site to make a Line Graph of the data listed above and turn it in here. e. Give a detailed CER writing response to the…arrow_forward
- 1. Several students performed this experiment without paying adequate attention to the details of the procedure. Briefly explain what effect each of the following procedural hanges would have on the size of the volume- to- temperature ratio calculated by the students. (a) One student failed to replenish the boiling water in the boiling- water bath as the flask was being heated. At the end of the 6 min of heating, the boiling water in the bath was only in contact with the lower portion of the flask. (b) Following the proper heating of the flask in the boiling water, a student removed the flask from the boiling- water bath but only partially immersed the flask in the ice-water bath during the cooling period. (c) A student neglected to close the pinch clamp before removing the flask from the boiling water bath and immersing it in the ice-water bath. (d) One student neglected to measure the volume of the flask before leaving the laboratory. Because the procedure called for a 125-mL…arrow_forwardAccording to Wikipedia, Radiocarbon dating (also referred to as carbon dating or carbon-14 dating) is a method for determining the age of an object containing organic material by using the properties of radiocarbon, a radioactive isotope of carbon. The method was developed in the late 1940s at the University of Chicago by Willard Libby, who received the Nobel Prize in Chemistry for his work in 1960. It is based on the fact that carbon- 14 is constantly being created in the atmosphere by the interaction of cosmic rays with atmospheric nitrogen. The resulting carbon-14 combines with atmospheric oxygen to form radioactive carbon dioxide, which is incorporated into plants by photosynthesis; animals then acquire carbon-14 by eating the plants. When the animal or plant dies, it stops exchanging carbon with its environment, and thereafter the amount of carbon-14 it contains begins to decrease as the carbon-14 undergoes radioactive decay. Measuring the amount of carbon-14 in a sample from a…arrow_forwardThe following volumes of 0.000300 M SCN are diluted to 15.00 mL. Determine the concentration of SCN in each sample after dilution. These values will be used during the experiment. To enter exponential values, use the format 1.0e-5. Sample 0.000300 M SCN (mL) [SCN'] (M) 1 1.50 3.50 7.00 4 10.00 3.arrow_forward
- Can you please please rewrite the question in your own words to clarify it, also write a useful hint to help you understand this problem and Provide the correct answer? Show the calculation and briefly describe the steps At the very end, write a paragraph addressing any knowledge/skill gaps, deficiencies in how you approach studying, and specifics on how you will improve Please answer fast and please try to answer allarrow_forwardNEED A HELP WITH THIS QUESTION 06arrow_forwardAnswer 2a and b pleasearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY